Chapter: Essentials of Anatomy and Physiology: Some Basic Chemistry
Organic Compounds of Importance
ORGANIC COMPOUNDS OF IMPORTANCE
Organic compounds all contain covalently bonded carbon and hydrogen atoms and perhaps other ele-ments as well. In the human body there are four major groups of organic compounds: carbohydrates, lipids, proteins, and nucleic acids.
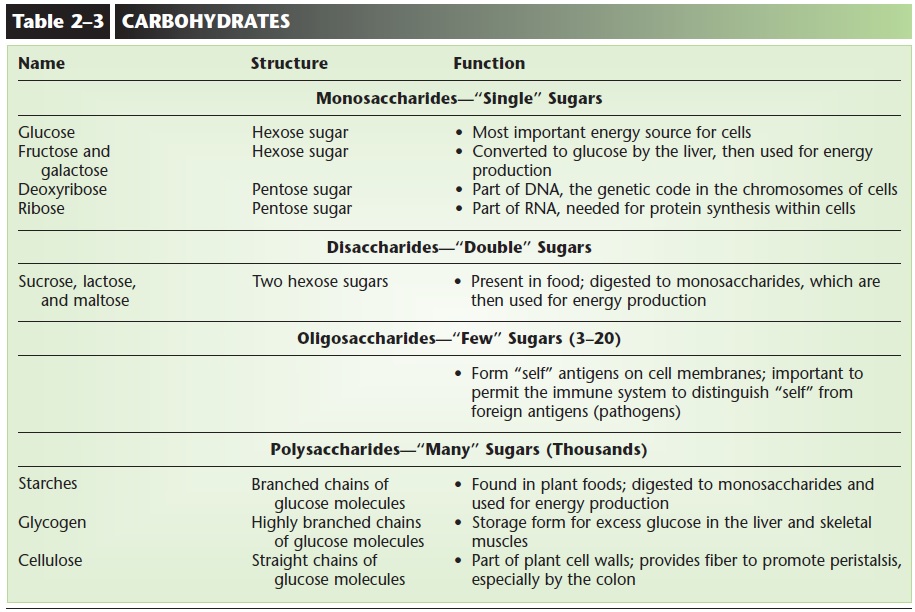
CARBOHYDRATES
A primary function of carbohydrates is to serve as sources of energy in cell respiration. All carbohydrates contain carbon, hydrogen, and oxygen and are classi-fied as monosaccharides, disaccharides, oligosaccha-rides, and polysaccharides. Saccharide means sugar, and the prefix indicates how many are present.
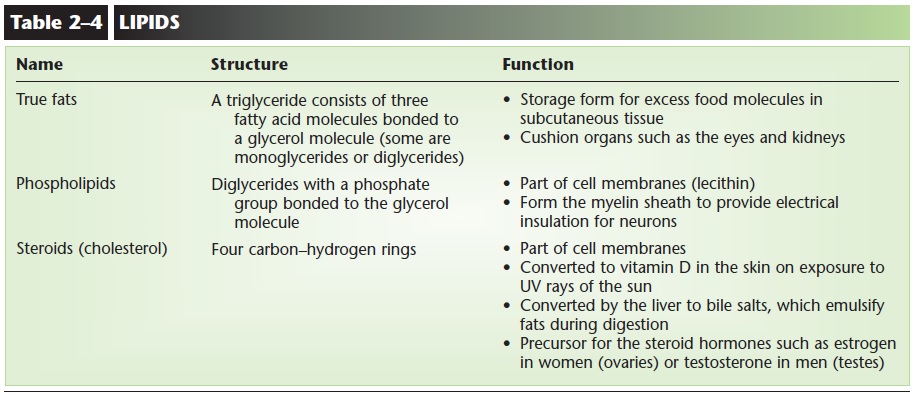
LIPIDS
Lipids contain the elements carbon, hydrogen, and oxygen; some also contain phosphorus. In this group of organic compounds are different types of sub-stances with very different functions. We will con-sider three types: true fats, phospholipids, and steroids (Fig. 2–7).
True fats (also called neutral fats) are made of one molecule of glycerol and one, two, or three fatty acid molecules. If three fatty acid molecules are bonded to a single glycerol, a triglyceride is formed. Two fatty acids and a glycerol form a diglyceride, and one fatty acid and a glycerol form a monoglyceride.
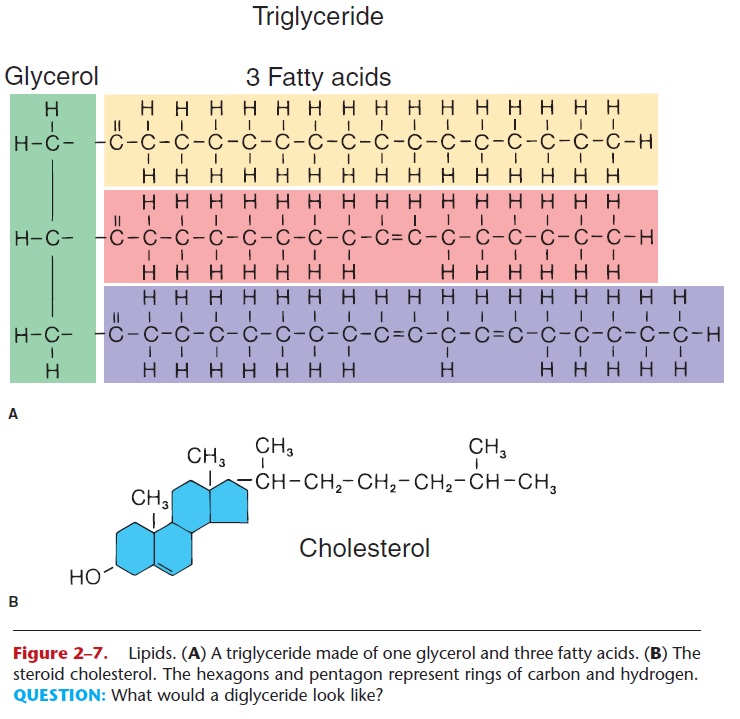
The fatty acids in a true fat may be saturated or unsaturated. Refer to Fig. 2–7 and notice that one of the fatty acids has single covalent bonds between all its carbon atoms. Each of these carbons is then bonded to the maximum number of hydrogens; this is a saturated fatty acid, meaning saturated with hydrogen. The other fatty acids shown have one or more (poly) dou-ble covalent bonds between their carbons and less than the maximum number of hydrogens; these are unsaturated fatty acids. Many triglycerides contain both saturated and unsaturated fatty acids, and though it is not as precise, it is often easier to speak of satu-rated and unsaturated fats, indicating the predomi-nance of one or the other type of fatty acid.
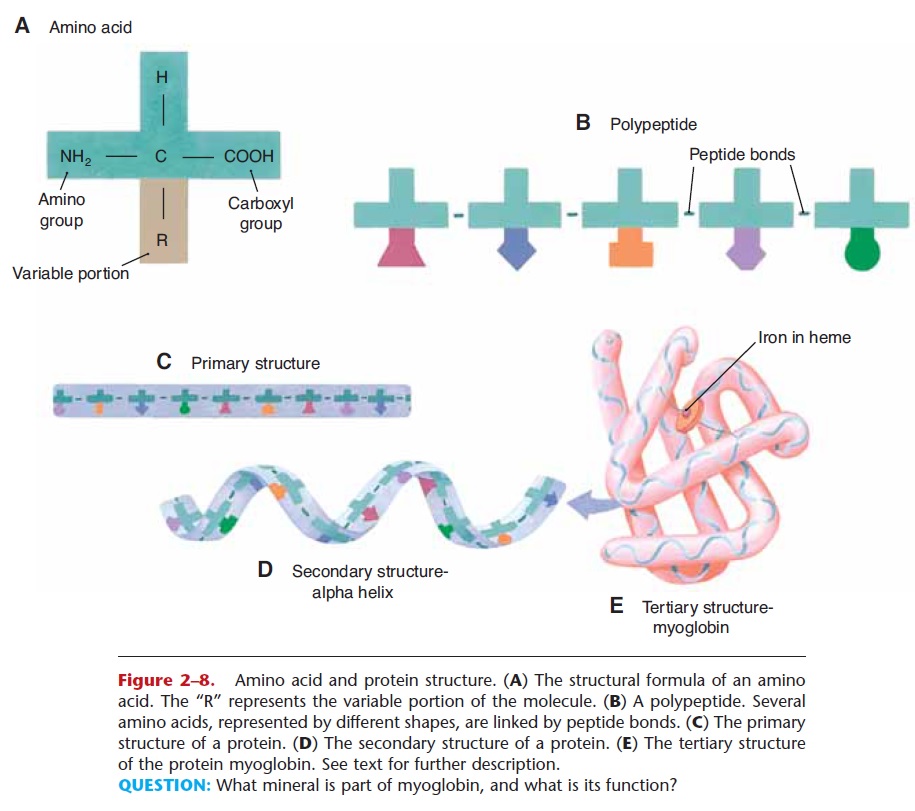
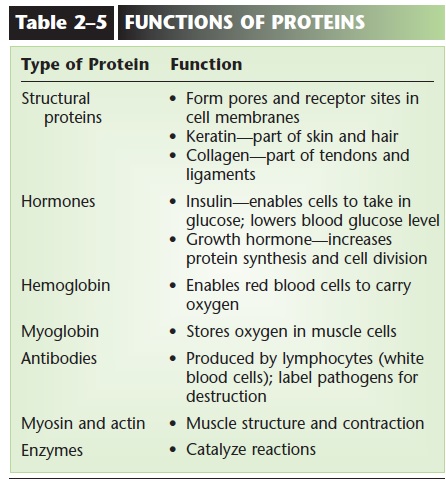
PROTEINS
Proteins are made of smaller subunits or building blocks called amino acids, which all contain the ele-ments carbon, hydrogen, oxygen, and nitrogen. Some amino acids contain sulfur, which permits the forma-tion of disulfide bonds. There are about 20 amino acids that make up human proteins. The structure of amino acids is shown in Fig. 2–8. Each amino acid has a central carbon atom covalently bonded to an atom of hydrogen, an amino group (NH2), and a carboxyl group (COOH). At the fourth bond of the central car-bon is the variable portion of the amino acid, repre-sented by R. The R group may be a single hydrogen atom, or a CH3 group, or a more complex configura-tion of carbon and hydrogen. This gives each of the 20 amino acids a slightly different physical shape. A bond between two amino acids is called a peptide bond, and a short chain of amino acids linked by peptide bonds is a polypeptide.
Enzymes
Enzymes are catalysts, which means that they speed up chemical reactions without the need for an external source of energy such as heat. The many reactions that take place within the body are catalyzed by spe-cific enzymes; all of these reactions must take place at body temperature.
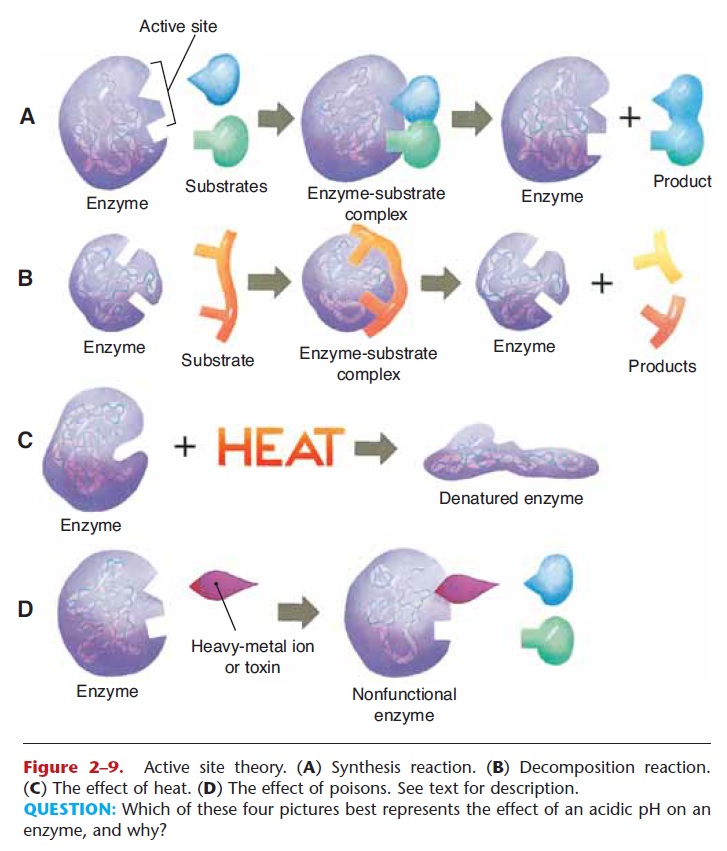
The way in which enzymes function as catalysts is called the active site theory, and is based on the shape of the enzyme and the shapes of the reacting molecules, called substrates. A simple synthesis reac-tion is depicted in Fig. 2–9A. Notice that the enzyme has a specific shape, as do the substrate molecules.
NUCLEIC ACIDS
DNA and RNA
The nucleic acids, DNA (deoxyribonucleic acid) and RNA (ribonucleic acid), are large molecules made of smaller subunits called nucleotides. A nucleotide con-sists of a pentose sugar, a phosphate group, and one of several nitrogenous bases. In DNA nucleotides, the sugar is deoxyribose, and the bases are adenine, gua-nine, cytosine, or thymine. In RNA nucleotides, the sugar is ribose, and the bases are adenine, guanine, cytosine, or uracil. DNA and RNA molecules are shown in Fig. 2–10. Notice that DNA looks somewhat like a twisted ladder; this ladder is two strands of
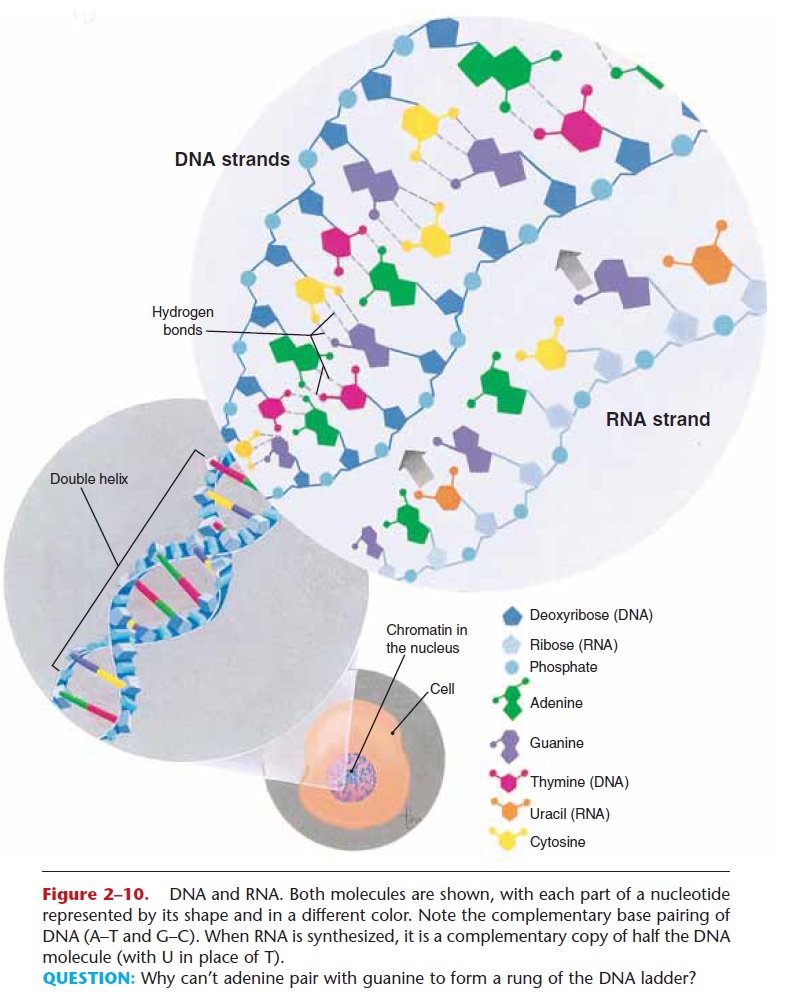
Figure 2–10. DNA and RNA. Both molecules are shown, with each part of a nucleotide represented by its shape and in a different color. Note the complementary base pairing of DNA (A–T and G–C). When RNA is synthesized, it is a complementary copy of half the DNA molecule (with U in place of T).
QUESTION: Why can’t adenine pair with guanine to form a rung of the DNA ladder?
nucleotides called a double helix (two coils). Alterna-ting phosphate and sugar molecules form the uprights of the ladder, and pairs of nitrogenous bases form the rungs. The size of the bases and the number of hydro-gen bonds each can form the complementary base pairing of the nucleic acids. In DNA, adenine is always paired with thymine (with two hydrogen bonds), and guanine is always paired with cytosine (with three hydrogen bonds).
ATP
ATP (adenosine triphosphate) is a specialized nucleotide that consists of the base adenine, the sugar ribose, and three phosphate groups. Mention has already been made of ATP as a product of cell respira- tion that contains biologically useful energy. ATP is one of several “energy transfer” molecules within cells, transferring the potential energy in food mole-cules to cell processes. When a molecule of glucose is broken down into carbon dioxide and water with the release of energy, the cell uses some of this energy to synthesize ATP. Present in cells are molecules of ADP (adenosine diphosphate) and phosphate. The energy released from glucose is used to loosely bond a third phosphate to ADP, forming ATP. When the bond of this third phosphate is again broken and energy is released, ATP then becomes the energy source for cell processes such as mitosis.
Related Topics