Chapter: Medical Surgical Nursing: Fluid and Electrolytes: Balance and Distribution
Potassium Deficit (Hypokalemia)
POTASSIUM
DEFICIT (HYPOKALEMIA)
Hypokalemia
(below-normal serum potassium concentration) usually indicates an actual
deficit in total potassium stores. Hypo-kalemia may occur in patients with
normal potassium stores; however, when alkalosis is present, a temporary shift
of serum potassium into the cells occurs.
As stated earlier,
hypokalemia is a common imbalance (Gennari, 1998). GI loss of potassium is
probably the most common cause of potassium depletion. Vomiting and gastric
suction fre-quently lead to hypokalemia, partly because potassium is actu-ally
lost when gastric fluid is lost, but more so because potassium is lost through
the kidneys in association with metabolic alka-losis. Because relatively large
amounts of potassium are con-tained in intestinal fluids, potassium deficit
occurs frequently with diarrhea. Intestinal fluid may contain as much potassium
as 30 mEq/L. Potassium deficit also occurs from prolonged intes-tinal
suctioning, recent ileostomy, and villous adenoma (a tumor of the intestinal
tract characterized by excretion of potassium-rich mucus).
Alterations in acid–base balance have a significant effect on potassium
distribution. The mechanism involves shifts of hydro-gen and potassium ions
between the cells and the ECF. Hypo-kalemia can cause alkalosis, and in turn
alkalosis can cause hypokalemia. For example, hydrogen ions move out of the
cells in alkalotic states to help correct the high pH, and potassium ions move
in to maintain an electrically neutral state. (This is dis-cussed further in
the section on acid–base balance.)
Hyperaldosteronism increases renal potassium wasting and can lead to
severe potassium depletion. Primary hyperaldosteronism is seen in patients with
adrenal adenomas. Secondary hyperaldo-steronism occurs in patients with
cirrhosis, nephrotic syndrome, heart failure, and malignant hypertension
(Wilcox, 1999).Potassium-losing diuretics, such as the thiazides (eg,
chlorothi-azide [Diuril] and polythiazide [Renese]), can induce hypokalemia,
particularly when administered in large doses to patients with inadequate
potassium intake. Other medications that can lead to hypokalemia include
corticosteroids, sodium penicillin, carbeni-cillin, and amphotericin B (Cohn et
al., 2000; Gennari, 1998).
Because insulin promotes
the entry of potassium into skeletal muscle and hepatic cells, patients with
persistent insulin hyper-secretion may experience hypokalemia, which is often
the case in patients receiving high-carbohydrate parenteral fluids (as in
par-enteral nutrition).
Patients who are unable
or unwilling to eat a normal diet for a prolonged period are at risk for
hypokalemia. This may occur in debilitated elderly people, alcoholics, and
patients with anorexia nervosa. In addition to poor intake, people with bulimia
fre-quently suffer increased potassium loss through self-induced vom-iting and
laxative and diuretic abuse.
Magnesium depletion
causes renal potassium loss and must be corrected first; otherwise, urine loss
of potassium will continue. Penicillins may produce renal potassium loss by
acting as poorly reabsorbable anions and thus increasing distal sodium delivery
and sodium-potassium loss.
Clinical Manifestations
Potassium deficiency can result in widespread derangements in
phys-iologic function. Severe hypokalemia can cause death through car-diac or
respiratory arrest. Clinical signs rarely develop before the serum potassium
level has fallen below 3 mEq/L (3 mmol/L) un-less the rate of fall has been
rapid. Manifestations of hypokalemia include fatigue, anorexia, nausea,
vomiting, muscle weakness, leg cramps, decreased bowel motility, paresthesias
(numbness and tingling), dysrhythmias, and increased sensitivity to digitalis
(Gennari, 1998). If prolonged, hypokalemia can lead to an inability of the
kidneys to concentrate urine, causing dilute urine (resulting in poly-uria,
nocturia) and excessive thirst. Potassium depletion depresses the release of
insulin and results in glucose intolerance.
Assessment and Diagnostic Findings
In hypokalemia, the
serum potassium concentration is less than the lower limit of normal.
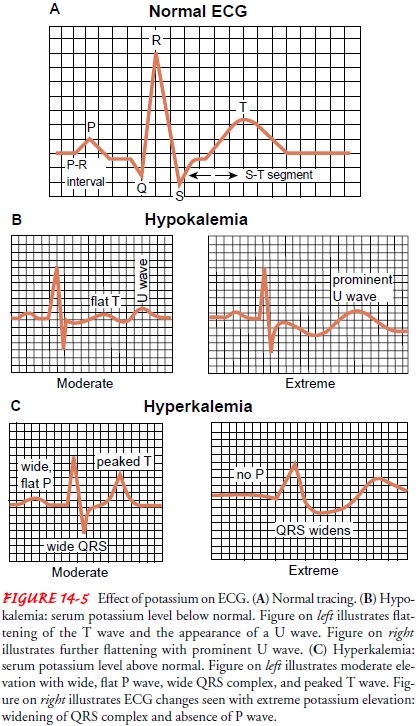
Electrocardiographic (ECG) changes can include flat T waves and/or inverted T
waves, suggesting ischemia, and depressed ST segments (Fig. 14-5). An elevated
U wave is specific to hypokalemia. Hypokalemia increases sensi-tivity to digitalis,
predisposing the patient to digitalis toxicity at lower digitalis levels.
Metabolic alkalosis is commonly associated with hypokalemia. This is discussed
further in the section on acid–base disturbances.
The source of the potassium loss is usually evident from a care-ful
history. When this is not the case, however, and the etiology of the loss is
unclear, a 24-hour urinary potassium excretion test can be performed to
distinguish between renal and extrarenal loss. Urinary potassium excretion
exceeding 20 mEq/24 h with hypo-kalemia suggests that renal potassium loss is
the cause.
Medical Management
If hypokalemia cannot be prevented by conventional measures such as increased intake in the daily diet, it is treated with oral or IV replacement therapy (Gennari, 1998). Potassium loss must be corrected daily; administration of 40 to 80 mEq/day of potassium is adequate in the adult if there are no abnormal losses of potassium.
For patients at risk for hypokalemia, a diet containing sufficient
potassium should be provided. Dietary intake of potassium in the average adult
is 50 to 100 mEq/day. Foods high in potassium in-clude fruits (especially
raisins, bananas, apricots, and oranges), vegetables, legumes, whole grains,
milk, and meat.
When dietary intake is
inadequate for any reason, the physi-cian may prescribe oral or IV potassium
supplements (Gennari, 1998). Many salt substitutes contain 50 to 60 mEq of
potassium per teaspoon and may be sufficient to prevent hypokalemia.When oral
administration of potassium is not feasible, the IV route is indicated. The IV
route is mandatory for patients with severe hypokalemia (eg, a serum level of 2
mEq/L). Although potassium chloride is usually used to correct
potassium deficits, the physician may prescribe potassium acetate or potassium
phosphate.
Nursing Management
Because hypokalemia can be life-threatening, the nurse needs to monitor
for its early presence in patients at risk. Fatigue, anorexia, muscle weakness,
decreased bowel motility, paresthesias, and dys-rhythmias are signals that
warrant assessing the serum potassium concentration. When available, the ECG
may provide useful in-formation. For example, patients receiving digitalis who
are at risk for potassium deficiency should be monitored closely for signs of
digitalis toxicity, because hypokalemia potentiates the action of digitalis.
Physicians usually prefer to keep the serum potassium level above 3.5 mEq/L
(3.5 mmol/L) in patients receiving digi-talis medications such as digoxin.
PREVENTING HYPOKALEMIA
Measures are taken to prevent hypokalemia when possible (Gen-nari,
1998). Prevention may involve encouraging the patient at risk to eat foods rich
in potassium (when the diet allows). Sources of potassium include fruit and
fruit juices (bananas, melon, citrus fruit), fresh and frozen vegetables, fresh
meats, and processed foods. When hypokalemia is due to abuse of laxatives or
diuretics, patient education may help alleviate the problem. Part of the health
his-tory and assessment should be directed at identifying problems amenable to
prevention through education. Careful monitoring of fluid intake and output is
necessary because 40 mEq of potassium is lost for every liter of urine output.
The ECG is monitored for changes, and arterial blood gas values are checked for
elevated bicarbonate and pH levels.
CORRECTING HYPOKALEMIA
Great care should be exercised when administering potassium,
particularly in older adults, who have lower lean body mass and total body
potassium levels and therefore lower potassium re-quirements. Additionally,
with the physiologic loss of renal func-tion with advancing years, potassium
may be retained more readily in older than in younger people.
ADMINISTERING IV POTASSIUM
Potassium should be
administered only after adequate urine flow has been established. A decrease in
urine volume to less than 20 mL/h for 2 consecutive hours is an indication to
stop the potassium infusion until the situation is evaluated. Potassium is
primarily excreted by the kidneys; therefore, when oliguria occurs, potassium
administration can cause the serum potassium concen-tration to rise
dangerously.
Each health care
facility has its own standard of care, which should be consulted; however, IV
potassium should not be ad-ministered faster than 20 mEq/h or in concentrations
greater than 30 to 40 mEq/L unless hypokalemia is severe, because this can
cause life-threatening dysrhythmias. When prepared for IV infusions, the fluid
should be agitated well to prevent bolus doses that can result when the potassium
concentrates at the bottom of the IV container.
When potassium is administered through a peripheral vein, the rate of
administration must be decreased to avoid irritating the vein and causing a
burning sensation during administration. In general, concentrations greater
than 60 mEq/L are not adminis-tered in peripheral veins because venous pain and
sclerosis may occur. For routine maintenance needs, potassium is suitably
di-luted and administered at a rate no faster than 10 mEq/h. In crit-ical
situations, more concentrated solutions (such as 40 mEq/L) may be administered
through a central line. Even in extreme hypo-kalemia, however, potassium should
be administered no faster than 20 to 40 mEq/h (suitably diluted). In such a
situation, the patient must be monitored by ECG and observed closely for other
signs and symptoms, such as changes in muscle strength.
Related Topics