Chapter: Medical Surgical Nursing: Fluid and Electrolytes: Balance and Distribution
Amount and Composition of Body Fluids
Fundamental Concepts
The nurse needs to
understand the physiology of fluid and electrolyte balance and acid–base
balance to anticipate, identify, and respond to possible imbalances in each.
The nurse also must use effective teaching and communication skills to help
prevent and treat various fluid and electrolyte disturbances.
AMOUNT
AND COMPOSITION OF BODY FLUIDS
Approximately 60% of a typical adult’s weight consists of fluid (water and
electrolytes). Factors that influence the amount of body fluid are age, gender,
and body fat. In general, younger people have a higher percentage of body fluid
than older people, and men have proportionately more body fluid than women.
Obese people have less fluid than thin people because fat cells contain little
water.
Body fluid is located in two fluid compartments: the intra-cellular
space (fluid in the cells) and the extracellular space (fluid outside the
cells). Approximately two thirds of body fluid is in the intracellular fluid
(ICF) compartment and is located primarily in the skeletal muscle mass.
The extracellular fluid (ECF) compartment is further divided into the
intravascular, interstitial, and transcellular fluid spaces. The intravascular
space (the fluid within the blood vessels) contains plasma. Approximately 3 L
of the average 6 L of blood volume is made up of plasma. The remaining 3 L is
made up of erythrocytes, leukocytes, and thrombocytes. The interstitial space
contains the fluid that surrounds the cell and totals about 11 to 12 L in an
adult. Lymph is an example of interstitial fluid. The transcellular space is
the smallest division of the ECF compartment and contains approximately 1 L of
fluid at any given time. Examples of transcellular fluid are cerebrospinal,
pericardial, synovial, intraocular, and pleural fluids; sweat; and digestive
secretions.
Body fluid normally shifts between the two major compart-ments or spaces
in an effort to maintain an equilibrium between the spaces. Loss of fluid from
the body can disrupt this equilibrium. Sometimes fluid is not lost from the
body but is unavailable for use by either the ICF or ECF. Loss of ECF into a
space that does not contribute to equilibrium between the ICF and the ECF is
referred to as a third-space fluid shift, or “third spacing” for short.
An early clue of a third-space fluid shift is a decrease in urine output
despite adequate fluid intake. Urine output decreases be-cause fluid shifts out
of the intravascular space; the kidneys then receive less blood and attempt to
compensate by decreasing urine output. Other signs and symptoms of third
spacing that indicate an intravascular fluid volume deficit include increased
heart rate, decreased blood pressure, decreased central venous pressure, edema,
increased body weight, and imbalances in fluid intake and output (I&O).
Third-space shifts occur in ascites,burns, peritonitis, bowel obstruction, and
massive bleeding into a joint or body cavity.
Electrolytes
Electrolytes in body fluids are active chemicals (cations, whichcarry
positive charges, and anions, which carry negative charges).The major cations
in body fluid are sodium, potassium, calcium,magnesium, and hydrogen ions. The
major anions are chloride,bicarbonate, phosphate, sulfate, and proteinate ions.
These chemicals unite in varying combinations. Therefore,electrolyte
concentration in the body is expressed in terms of milliequivalents (mEq) per
liter, a measure of chemical activity, rather than in terms of milligrams (mg),
a unit of weight. More specifically, a milliequivalent is defined as being
equivalent to the electrochemical activity of 1 mg of hydrogen. In a solution,
cations and anions are equal in mEq/L.
Electrolyte concentrations in the ICF differ from those in the ECF, as reflected in Table 14-1. Because special techniques are required to measure electrolyte concentrations in the ICF, it is customary to measure the electrolytes in the most accessible por-tion of the ECF, namely the plasma.
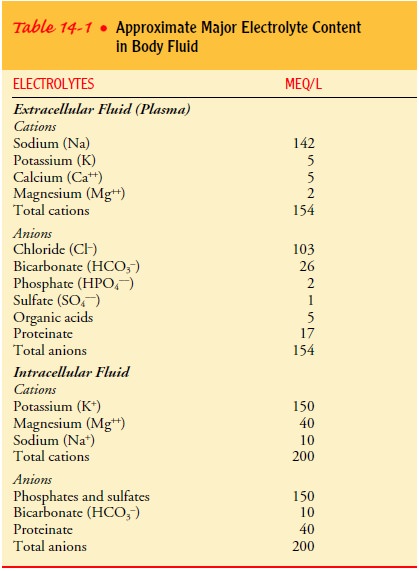
Sodium ions, which are positively charged, far outnumber the other
cations in the ECF. Because sodium concentration affects the overall
concentration of the ECF, sodium is important in reg-ulating the volume of body
fluid. Retention of sodium is associ-ated with fluid retention, and excessive
loss of sodium is usually associated with decreased volume of body fluid.
As shown in Table 14-1,
the major electrolytes in the ICF are potassium and phosphate. The ECF has a
low concentration of potassium and can tolerate only small changes in potassium
con-centrations. Therefore, release of large stores of intracellular potassium,
typically caused by trauma to the cells and tissues, can be extremely
dangerous.
The body expends a great
deal of energy maintaining the high extracellular concentration of sodium and
the high intracellular concentration of potassium. It does so by means of cell
mem-brane pumps that exchange sodium and potassium ions. Normal movement of
fluids through the capillary wall into the tissues de-pends on hydrostatic pressure (the pressure
exerted by the fluid on the walls of the blood vessel) at both the arterial and
the ve-nous ends of the vessel and the osmotic pressure exerted by the protein
of plasma. The direction of fluid movement depends on the differences in these
two opposing forces (hydrostatic versus osmotic pressure).
In addition to electrolytes, the ECF transports other sub-stances, such
as enzymes and hormones. It also carries blood com-ponents, such as red and
white blood cells, throughout the body.
Related Topics