Chapter: Plant Biochemistry: The use of energy from sunlight by photosynthesis is the basis of life on earth
Phycobilisomes enable cyanobacteria and red algae to carry out photosynthesis even in dim light
Phycobilisomes enable cyanobacteria and red algae to carry out photosynthesis even in dim light
Cyanobacteria and red algae possess antennae structures that can collect light of very low intensity. These antennae are arranged as complexes on top of the membrane near the reaction centers of photosystem II (Fig. 2.14).
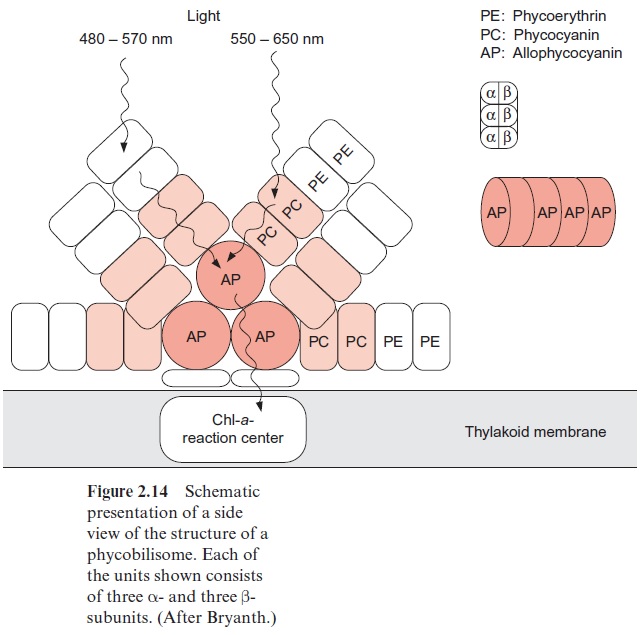
These complexes, termed phycobilisomes, consist of proteins (phyco-biliproteins), which are covalently linked with phycobilins.Phycobilins are open-chained tetrapyrroles and therefore are structurally related to the chlorophylls. Open-chained tetrapyrroles are also contained in bile, which explains the name -bilin. The phycobilins are linked to the protein by a thioether bond between an SH-group of the protein and the vinyl side chain of the phycobilin. The protein phycoerythrin is linked to the chromophorephycoerythrobilin, and the proteins phycocyanin and allophycocyanin to the chromophore phycocyanobilin (Fig. 2.15). The basic structure in the phyco-biliproteins consists of a heterodimer composed of α- and β-subunits. Each of these protein subunits binds one to four phycobilins as a chromophore. Three of these heterodimers aggregate to a trimer ( α‚β )3 and thus form the actual building block of a phycobilisome. Specific linker polypeptides func-tion as “mortar” between the building blocks.
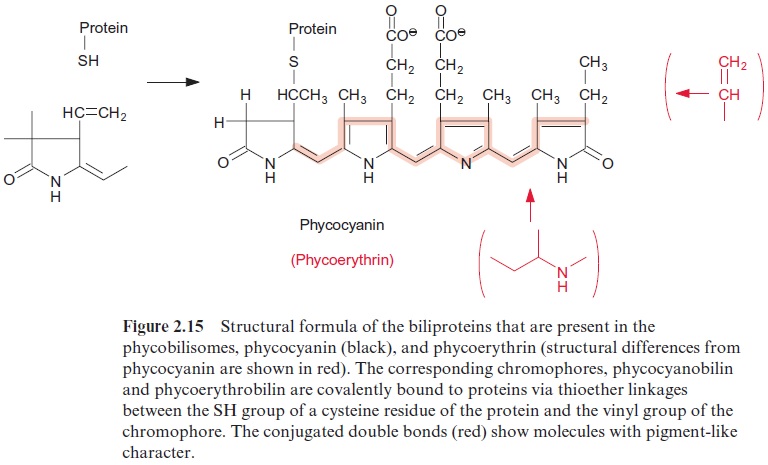
Figure 2.14 shows the structure of a phycobilisome. The phycobilisome is attached to the membrane by anchor proteins. Three aggregates of four to five ( α,β )3 units form the core. This core contains the chromophore allo-phycocyanin (AP) to which cylindrical rod like structures are attached, each with four to six building blocks. The inner units contain mainly phycocyanine (PC) and the outer ones phycoerythrin (PE). The function of this structural organization is illustrated by the absorption spectra of the various biliproteins shown in Figure 2.16. The light of shorter wave-length is absorbed in the periphery of the rods by phycoerythrin and the light of longer wavelength in the inner regions of the rods by phycocyanin. The core transfers the excitons to the reaction center. The principle of spa-tial distribution between the short wavelength absorbing pigments at the periphery and the long wavelength absorbing pigments in the center is also implemented for the PS II antennae of higher plants (Fig. 2.10).
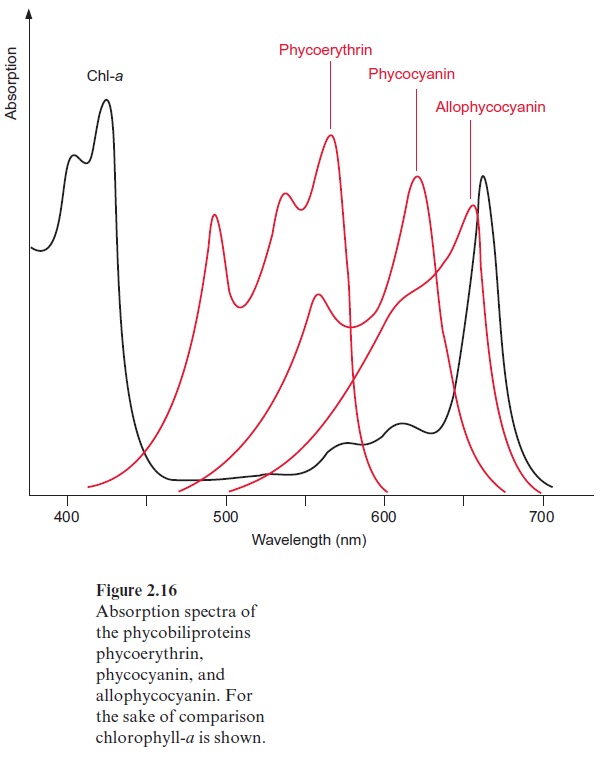
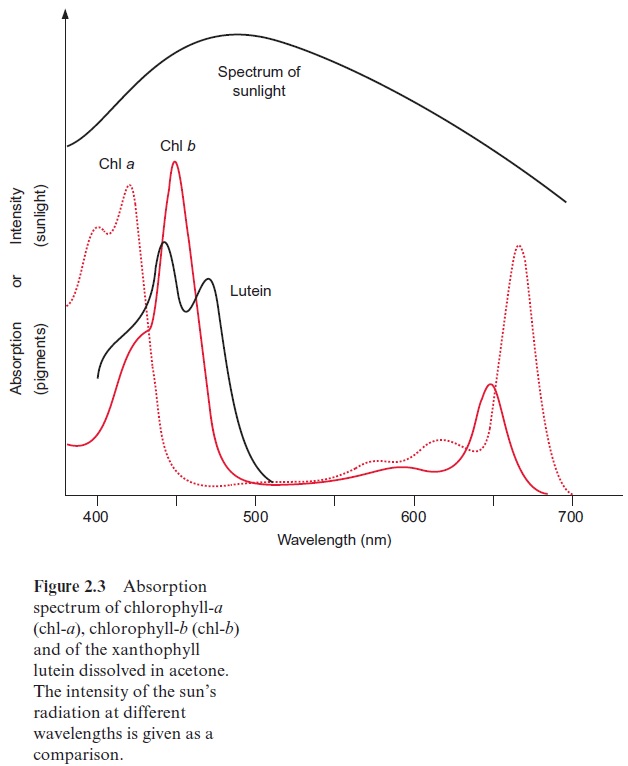
Due to the phycobiliproteins, phycobilisomes are able to absorb green light very efficiently (Fig. 2.16), thus allowing cyanobacteria and red algae to survive in deep waters with low light intensities. At these depths, due to the “green window” of photosynthesis (Fig. 2.3), only green light is avail-able, as the light of the other wavelengths is absorbed by green algae living in the upper regions of the water column. The algae in the deeper regions are obliged to invest a large portion of their cellular matter in phycobili-somes in order to carry out photosynthesis at this very low light intensity and at distinct wavelengths. Biliproteins can amount to 40% of the total cellular protein of the algae. These organisms undertake an extraordinary expenditure to collect enough light for survival.
Related Topics