Chapter: Plant Biochemistry: The use of energy from sunlight by photosynthesis is the basis of life on earth
Light absorption excites the chlorophyll molecule
Light absorption excites the chlorophyll molecule
What happens when a chromophore absorbs a photon? When a photon with a certain wavelength hits a chromophore molecule that absorbs light of this wavelength, the energy of the photon excites electrons and trans-fers them to a higher energy level. This occurs as an “all or nothing” proc-ess. According to the principle of energy conservation expressed by the first law of thermodynamics, the energy of the chromophore is increased by the energy of the photon, which results in an excited state of the chromo-phore molecule. The energy is absorbed only in discrete quanta, resulting in discrete excitation states. The energy required to excite a chromophore molecule depends on the chromophore structure. A general property of chromophores is that they contain many conjugated double bonds, 10 in the case of the tetrapyrrole ring of chl-a. These double bonds are delocalized. Figure 2.5 shows two possible resonance forms.
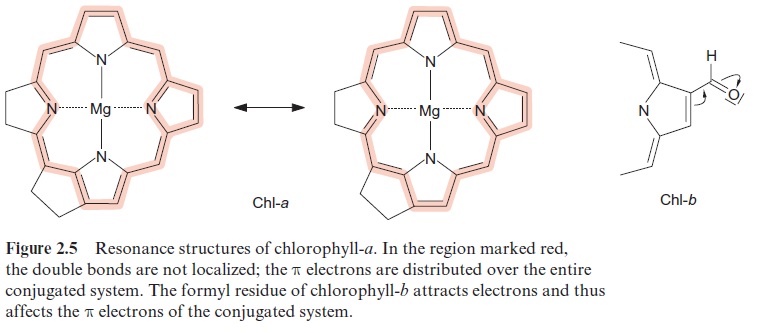
After absorption of energy, an electron of the conjugated system is ele-vated to a higher orbit. This excitation state is termed singlet.Figure 2.6 shows a scheme of the excitation process. As a rule, the higher the number of double bonds in the conjugated system, the lower the amount of energy required to produce a first singlet state. For the excitation of chlorophyll, dark red light is sufficient, whereas butadiene, with only two conjugated double bonds, requires energy-rich ultraviolet light for excitation. The light absorption of the conjugated system of the tetrapyrrole ring is influenced by the side chains. Thus, the differences in the absorption maxima of chl-a and chl-b mentioned previously can be explained by an electron attracting effect of the carbonyl side chain in ring b of chl-b(Fig. 2.5).
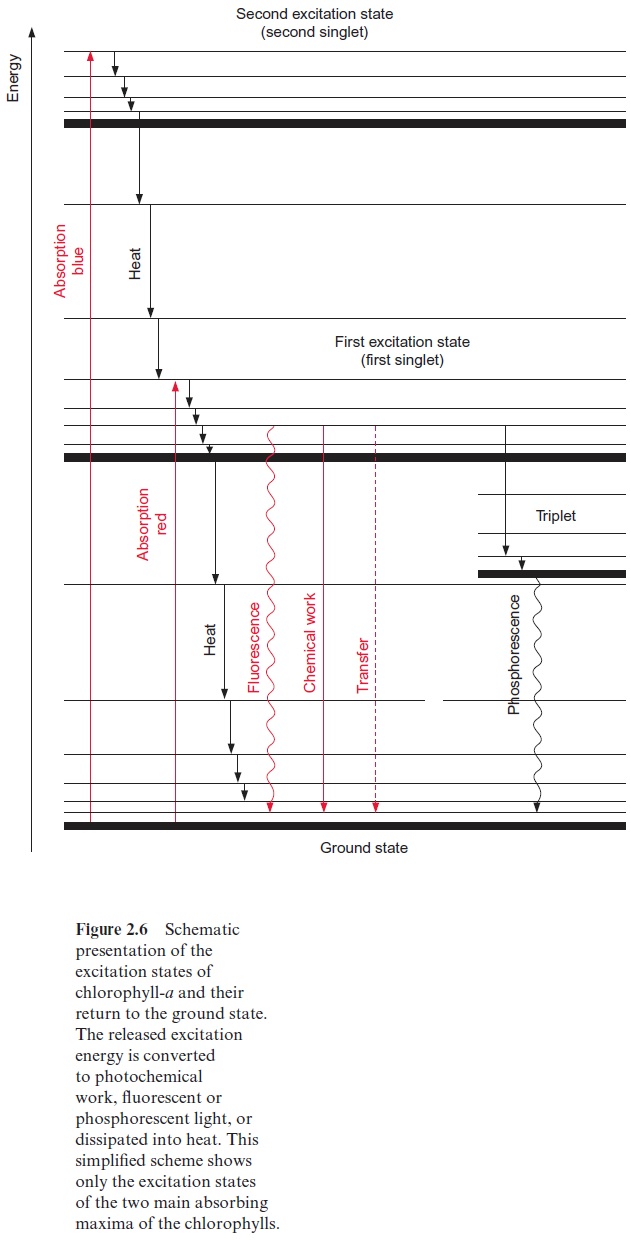
The spectra of chl-a and chl-b (Fig. 2.3) each have two main absorption maxima, showing that each chlorophyll has two main excitation states. In addition, chlorophylls have minor absorption maxima, which for the sake of simplicity will not be discussed here. The two main excitation states of chlo-rophyll are known as the first and second singlet (Fig. 2.6). The absorption maxima in the spectra are relatively broad. At a higher resolution the spec-tra can be shown to consist of many separate absorption lines. This fine structure of the absorption spectra is due to chlorophyll molecules that are in the ground and in the singlet states as well in rotationand vibration. In the energy scheme the various rotation and vibration energy levels are drawn as fine lines and the corresponding ground states as solid lines (Fig. 2.6).
The energy levels of the various rotation and vibration states of the ground state overlap with the lowest energy levels of the first singlet. Analogously, the energy levels of the first and the second singlet also over-lap. If a chlorophyll molecule absorbs light in the region of its absorption maximum (blue light), one of its electrons is elevated to the second singlet state. This second singlet state with a half-life of only 1012 s is too unsta-ble to use its energy for chemical work. The excited molecules lose energy as heat by rotations and vibrations until the first singlet state is reached. This first singlet state can also be attained by absorption of a photon of red light, which contains less energy. The first singlet state is much more stable than the second one; its half-life time is 4 · 109 s.
The return of the chlorophyll molecule from the first singlet state to the ground state can proceed in different ways:
1. The most important path for the conversion of the energy released upon the return of the first singlet state to the ground state is its utiliza-tion for chemical work. The chlorophyll molecule transfers the excited electron from the first singlet state to an electron acceptor and a pos-the excited electron is bound less strongly to the chromophore molecule than in the ground state. When the chlorophyll molecule returns to the ground state, the free energy derived from this process is conserved for chemical work. As an alternative, the electron deficit in the chl+. radical may be replenished by another electron donor (e.g., water).
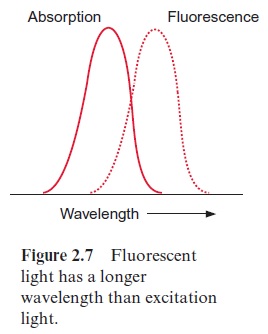
2. The excited chlorophyll can return to the ground state by releasing exci-tation energy as light; this emitted light is namedfluorescence. Due to vibrations and rotations, part of the excitation energy is usually lost as heat, with the result that the fluorescence light has less energy (corre-sponding to a longer wavelength) than the energy of the excitation light, which was required for attaining the first singlet state (Fig. 2.7).
3. It is also possible that the return from the first singlet to the ground state proceeds in a stepwise fashion via the various levels of vibration and rotation energy, by which the energy difference is completely con-verted into heat.
4. By releasing part of the excitation energy as heat, the chlorophyll mol-ecule can attain a lower energy excitation state, called the first triplet state. This triplet state cannot be reached directly from the ground state by excitation, since the spin of the excited electrons has been reversed. Since the probability of a reversal spin is low, the triplet state does not occur frequently. In the case of a very high excitation, however, some of the electrons of the chlorophyll molecules can reach this state. By emitting so-calledphosphorescent light, the molecule can return from the triplet state to the ground state. Phosphorescent light is lower in energy than the light required to attain the first singlet state. The return from the triplet state to the ground state requires a reversal of the electronspin. As this is rather improbable, the triplet state, in comparison to the first singlet state, has a relatively long half-life time (10 -4 to 10 -2 s). The triplet state of the chlorophyll has no function in photosynthesis per se. In its triplet state, however, the chlorophyll can excite oxygen to a sin-glet state, whereby the oxygen becomes very reactive (reactive oxygen species, ROS) with a damaging effect on cell constituents.
5. The return to the ground state can be coupled with the excitation of a neighboring chromophore molecule.
Related Topics