Chapter: Plant Biochemistry: The use of energy from sunlight by photosynthesis is the basis of life on earth
Pigments capture energy from sunlight
Pigments capture energy from sunlight
The energy content of light depends on its wavelength
In Berlin at the beginning of the twentieth century Max Planck and Albert Einstein, two Nobel Prize winners, carried out the epoch-making studies proving that light has a dual nature. It can be regarded as an electromagnetic wave as well as an emission of particles, which are termed light quanta or photons.
The energy of the photon is proportional to its frequency v:
E = h . v = h . c / λ
where h is the Planck constant (6.6 · 10-34 J s) and c the velocity of the light (3 · 108 m s-1). λ is the wavelength of light.
The mole (abbreviated to mol) is used as a chemical measure for the amount of molecules and the amount of photons corresponding to 6 · 1023 molecules or photons (Avogadro number NA). The energy of one mol photons amounts to:
E = h ⋅ c / λ ⋅ NA (2.2)
In order to utilize the energy of a photon in a thermodynamic sense, this energy must be at least as high as the Gibbs free energy of the photochemi-cal reaction involved. (In fact much energy is lost during energy conversion, with the consequence that the energy of the photon must be higher than the Gibbs free energy of the corresponding reaction.) We can equate the Gibbs free energy ∆G with the energy of the absorbed light:
ΔG = E = h ⋅ c / λ ⋅ NA (2.3)
The introduction of numerical values of the constants h, c, and NA yields:
ΔG = 6.6 . 10-34 . (J . s) . 3 . 108(m) / (s) . 1 / λ(m) . 6.1023 / (mol)
ΔG = 119000 / λ(nm) [kJ/mol photons]
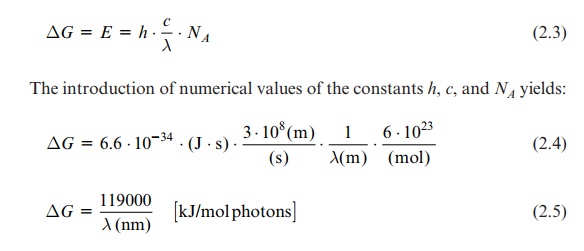
It is often useful to state the electrical potential ( ∆E) of the irradiation instead of energy when comparing photosynthetic reactions with redox reactions:
ΔE = - ΔG / F
where F = number of charges per mol = 96,485 Amp · s · mol-1. The
introduction of this value yields:
ΔE = - NA . . h . c / F.λ(nm) = 1231 / λ(nm) [ Volt]
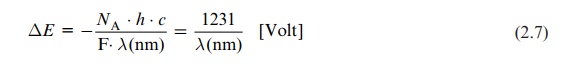
The human eye perceives only the small range between about 400 and 700 nm of the broad spectrum of electromagnetic waves (Fig. 2.2). The light in this range, where the intensity of solar radiation is especially high, is uti-lized in plant photosynthesis. Bacterial photosynthesis, however, is able to utilize light in the infrared range.
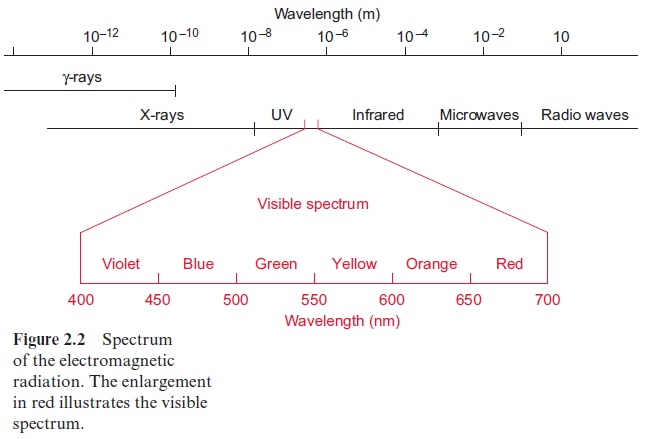
According to equation 2.3 the energy of irradiated light is inversely proportional to the wavelength. Table 2.1 shows the light energy per mol photons for light of different colors. Consequently, violet light has an energy of about 300 kJ/mol photons. Dark blue light, with the highest wavelength (700 nm) that can still be utilized by plant photosynthesis, contains 170 kJ/ mol photons. This is only about half the energy content of violet light.
Chlorophyll is the main photosynthetic pigment
In photosynthesis of a green plant, light is collected primarily by chlorophylls, pigments that absorb light at a wavelength below 480 nm and between 550 and 700 nm (Fig. 2.3). When white sunlight falls on a chlorophyll layer, the green light with a wavelength between 480 and 550 nm is not absorbed, but is reflected. This is why plant chlorophylls and whole leaves appear green.
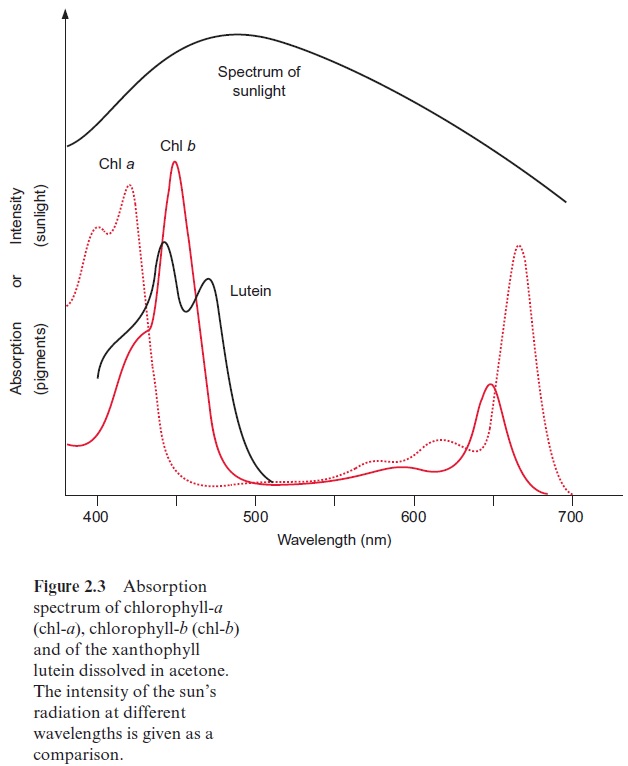
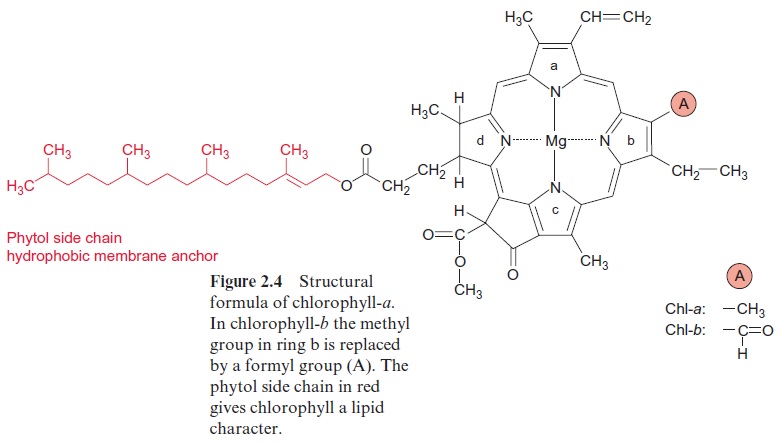
Experiments carried out between 1905 and 1913 in Zurich and Berlin by Richard Willstätter and his collaborators led to the discovery of the structural formula of the green leaf pigment chlorophyll, a milestone in the history of chemistry. This discovery made such an impact that Richard Willstätter was awarded the Nobel Prize in Chemistry as early as 1915. There are different classes of chlorophylls. Figure 2.4 shows the structural formulas of chlorophyll-a and chlorophyll-b (chl-a, chl-b). The basic struc-ture is a ring made of four pyrroles, a tetrapyrrole, which is also named porphyrin. Mg++ is present in the center of the ring as the central atom. Mg++ is covalently bound with two N atoms and coordinately bound to the other two atoms of the tetrapyrrole ring. A cyclopentanone is attached to ring c. At ring d a propionic acid group forms an ester with the alcohol phytol. Phytol consists of a long branched hydrocarbon chain with one C-C double bond. It is derived from an isoprenoid, formed from four isoprene units . This long hydrophobic hydrocarbon tail renders the chlorophyll highly soluble in lipids and therefore promotes its presence in the membrane phase. Chlorophyll always occurs bound to proteins. Chl-b contains a formyl residue in ring b instead of the methyl residue as in chl-a. This small difference has a large influence on light absorption.Figure 2.3 shows that the absorption spectra of chl-a and chl-b differ markedly.
In plants, the ratio chl-a to chl-b is about three to one. Only chl-a is a constituent of the photosynthetic reaction centers and therefore it can be regarded as the central photosynthesis pigment. In a wide range of the visible spectrum, however, chl-a does not absorb light (Fig. 2.3). This non-absorbing region is named the “green window.” The absorption gap is narrowed by the light absorption of chl-b, with its first maximum at a higher wavelength than chl-a and the second maximum at a lower wavelength. The light energy absorbed by chl-b can be transferred very efficiently to chl-a. In this way, chl-b enhances the plant’s efficiency for utilizing sunlight energy.
The structure of chlorophylls has remained remarkably constant during the course of evolution. Purple bacteria, probably formed more than 3 billion years ago, contain as photosynthetic pigment a bacteriochlorophyll-a, which differs from the chl-a shown in Fig. 2.4 only by the alteration of one side chain and by the lack of one double bond. This, however, influences light absorption; both absorption maxima are shifted outwards and the non-absorbing spectral region in the middle is broadened. This shift allows purple bacteria to utilize light in the infrared region.
The tetrapyrrole ring not only is a constituent of chlorophyll but also has attained a variety of other functions during evolution. It is involved in methane formation by bacteria with Ni++ as the central atom. With Co++ it forms cobalamin (vitamin B12), which participates as a cofactor in reac-tions in which hydrogen and organic groups change their position. With Fe++ instead of Mg++ as the central atom, the tetrapyrrole ring forms the basic structure of hemes (Fig. 3.24), which as cytochromes function as redox carriers in electron transport processes and as myoglobin or hemoglobin stores or transports oxygen in aerobic organ-isms. The tetrapyrrole ring in animal hemoglobin differs only slightly from the tetrapyrrole ring of chl-a (Fig. 2.4).
It seems remarkable that a substance that attained a certain function during evolution is being utilized after only minor changes for completely different functions. The reason for this functional variability is that the reactivity of compounds such as chlorophyll or heme is governed to a great extent by the proteins to which they are bound.
Chlorophyll molecules are bound to chlorophyll-binding proteins. In a complex with proteins the absorption spectrum of the bound chlorophyll differs considerably from the absorption spectrum of the free chlorophyll. The same applies for other light-absorbing compounds, such as carotenoids, xanthophylls, and phycobilins, which also occur bound to proteins. These complexes will be discussed in the following sections. For better discrimi-nation in this text book, free absorbing compounds are calledchromophore (Greek, carrier of color) and the chromophore-protein complexes are called pigments. Pigments are further characterized by the wavelength of theirabsorption maximum. Chlorophyll-a700 describes a pigment of protein-chl-a complex with an absorption maximum of 700 nm. Another common desig-nation is P700; this nomination leaves the nature of the chromophore open.
Related Topics