Chapter: Plant Biochemistry: The use of energy from sunlight by photosynthesis is the basis of life on earth
The energy content of light depends on its wavelength
The energy content of light depends on its wavelength
In Berlin at the beginning of the twentieth century Max Planck and Albert Einstein, two Nobel Prize winners, carried out the epoch-making studies proving that light has a dual nature. It can be regarded as an electromagnetic wave as well as an emission of particles, which are termed light quanta or photons.
The energy of the photon is proportional to its frequency v:
E = h . v = h . c / λ
where h is the Planck constant (6.6 · 10-34 J s) and c the velocity of the light (3 · 108 m s-1). λ is the wavelength of light.
The mole (abbreviated to mol) is used as a chemical measure for the amount of molecules and the amount of photons corresponding to 6 · 1023 molecules or photons (Avogadro number NA). The energy of one mol photons amounts to:
E = h ⋅ c / λ ⋅ NA (2.2)
In order to utilize the energy of a photon in a thermodynamic sense, this energy must be at least as high as the Gibbs free energy of the photochemi-cal reaction involved. (In fact much energy is lost during energy conversion, with the consequence that the energy of the photon must be higher than the Gibbs free energy of the corresponding reaction.) We can equate the Gibbs free energy ∆G with the energy of the absorbed light:
ΔG = E = h ⋅ c / λ ⋅ NA (2.3)
The introduction of numerical values of the constants h, c, and NA yields:
ΔG = 6.6 . 10-34 . (J . s) . 3 . 108(m) / (s) . 1 / λ(m) . 6.1023 / (mol)
ΔG = 119000 / λ(nm) [kJ/mol photons]
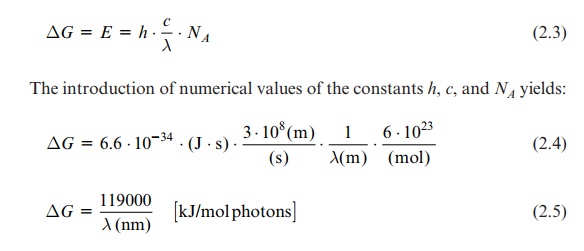
It is often useful to state the electrical potential ( ∆E) of the irradiation instead of energy when comparing photosynthetic reactions with redox reactions:
ΔE = - ΔG / F
where F = number of charges per mol = 96,485 Amp · s · mol-1. The
introduction of this value yields:
ΔE = - NA . . h . c / F.λ(nm) = 1231 / λ(nm) [ Volt]
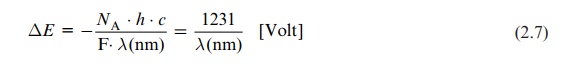
The human eye perceives only the small range between about 400 and 700 nm of the broad spectrum of electromagnetic waves (Fig. 2.2). The light in this range, where the intensity of solar radiation is especially high, is uti-lized in plant photosynthesis. Bacterial photosynthesis, however, is able to utilize light in the infrared range.
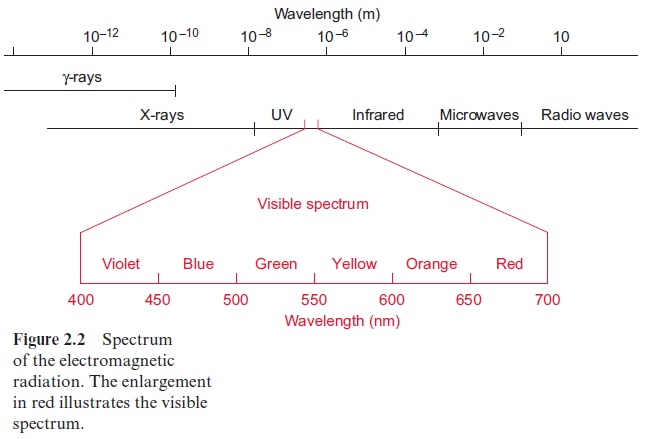
According to equation 2.3 the energy of irradiated light is inversely proportional to the wavelength. Table 2.1 shows the light energy per mol photons for light of different colors. Consequently, violet light has an energy of about 300 kJ/mol photons. Dark blue light, with the highest wavelength (700 nm) that can still be utilized by plant photosynthesis, contains 170 kJ/ mol photons. This is only about half the energy content of violet light.
Related Topics