Chapter: Microbiology and Immunology: Bacteriology: Nonsporing Anaerobes
Pathogenesis and Immunity - Escherichia coli
Pathogenesis and Immunity
E. coli is an invasive bacterium. It colonizes the human intestineand, under specific conditions, directly invades the intestinal mucosa or produces toxins to cause intestinal infections. The bacteria can enter the blood stream and cause septicemia, men-ingitis, and other systemic manifestations. The bacteria, under certain conditions, directly invade urinary tract causing UTIs or cause intra-abdominal infections.
◗ Virulence factors
E. coli produces several virulence factors (Table 31-5), whichinclude the following:
a) Common virulence factors associated with Enterobac-teriaceae.
b) Specialized virulence factors associated specifically with E. coli.
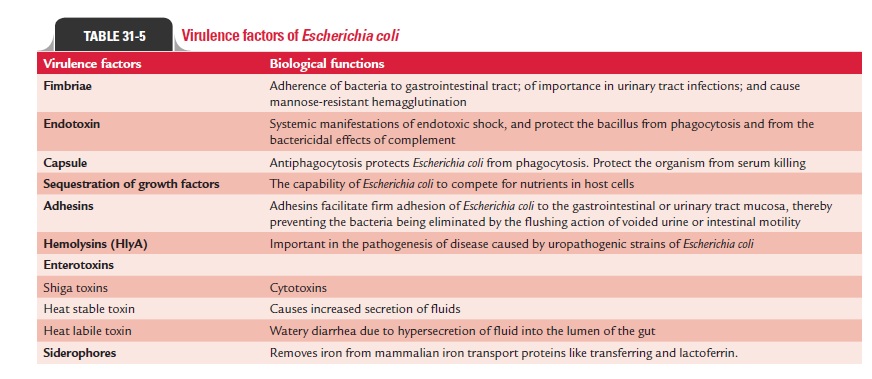
Common virulence factors associated with Enterobacteria-ceae: These include following factors: (a) fimbriae, (b) endo-toxin, (c) capsule, and (d) sequestration of growth factors.
Fimbriae: Fimbriae promote virulence ofE. coliand other mem-bers of the Enterobacteriaceae. The fimbriae are of two types:
· The first type is most common and is encoded by chromo-somes, but is not related with virulence of the bacteria;
· The second type of fimbriae is encoded by plasmids, found only in small numbers, but is closely related with virulence of the bacteria.
Some of them do not occur as morphologically distinct struc-tures but only as surface antigens. K88 and K99 antigens in E. colistrains causing diarrhea in animals or CFA in ETEC areexamples of such fimbriae. The fimbriae play an important role in pathogenesis of UTI caused by E. coli.
Endotoxin: Endotoxin is a major virulence factor sharedamong all aerobic and some anaerobic Gram-negative bacte-ria including E. coli. Endotoxin is responsible for many of the systemic manifestations of Gram-negative bacteria caused by E. coli infections. Endotoxin also protects the bacillus fromphagocytosis and from the bactericidal effects of complement.
Capsule: Hydrophilic capsular K antigens protectE. colifromphagocytosis, which repel the hydrophobic phagocytic cell surface. The capsular antigens interfere with the binding of antibodies to the bacteria. However, the capsule is not effective in the presence of antibody to O or K antigen. Most strains of E. coli responsible for neonatal meningitis and septicemia pos-sess the KI envelope antigen, a virulence factor similar to the group B antigen of meningococci. E. coli and other enteric bac-teria capable of producing systemic infections are frequently resistant to serum killing. Capsule of the bacterium protects the organism from serum killing.
Sequestration of growth factors: The capability of the bacteria tocompete for nutrients in host cells is an important property of virulent bacteria. E. coli and other enteric bacteria compete for iron, which is an important factor for their growth. The bacteria produce iron-chelating compounds, such as siderophores, entero-bactin, and aerobactin, which facilitates the adsorption of iron by bacteria. Also the bacteria produce hemolysins, which lyse host erythrocytes, thereby releasing iron compounds for use by bacteria.
Specialized virulence factors associated specifically with E. coli : These include adhesins and exotoxins.
Adhesins: E. coliorganisms possess numerous highly special-ized adhesins. These adhesins include (a) CFAs (CFA/I, CFA/ II, CFA/III), (b) aggregative adherence fimbriae (AAF/I, AAF/ II, AAF/III), (c) bundle-forming pili (Bfp), (d) intimin, (e) P pili (binds to P blood group antigens), (f) Ipa (invasion plasmid antigen) protein, and (g) Dr fimbriae (bind to Dr blood group antigens). All these adhesins facilitate firm adhesion of E. coli to the gastrointestinal or urinary tract mucosa, thereby pre-venting the bacteria being eliminated by the flushing action of voided urine or intestinal motility.
Exotoxins: E. colialso produces two types of exotoxins: (a) hemo-lysins (HlyA) and (b) enterotoxins. Hemolysins are consid-ered important in the pathogenesis of disease caused by uropathogenic strains of E. coli. Enterotoxins are important virulent factors of E.coli. Three distinct types of E. coli entero-toxins have been recognized. These include (a) Shiga toxins (Stx-1, Stx-2), (b) heat-stable toxins (STa and STb), and (c) heat-labile toxins (LT-I and LT-II).
Shiga toxins:Shiga toxin (Stx) is so named because it is similarto the Shigella dysenteriae type 1 toxin in its physical, antigenic, and biological properties. The toxin is also named verocyto-toxin or verotoxin (VT) because the toxin was first detected by its cytotoxic effect on Vero cells. Shiga toxins are of two types: Stx-1 and Stx-2. Both toxins are encoded by lysogenic bacterio-phages. Both have one A subunit and five B subunits. Subunit B binds to a specific glycolipid (globotriaosylceramide, GbJ) present on the host cell. Both the toxins, although show same biological activity, are antigenically different. Stx-2 is not neu-tralized by the antibodies produced against Stx, unlike Stx-1. Shiga toxins demonstrate cytotoxicity activities in vero or HeLa cells. The toxin also shows enterotoxicity in rabbit ileal loops and paralytic lethality in mouse.
Heat-stable toxin:Heat-stable toxins (ST) are low-molecular-weight proteins and are of two types: STa and STb. STa is associated with disease in humans. STa is a small, methanol soluble, monomeric toxin that acts by activation of cyclic gua-nosine monophosphate (cGMP) in the intestine. STa binds to guanylate cyclase leading to an increase in the level of cGMP and subsequent increased secretion of fluids. The toxin acts very rapidly and causes accumulation of fluid in the intestines of infant mice within 4 hours of intragastric administration; hence infant mouse is a frequently used animal model for dem-onstration of STa. The toxin also causes accumulation of fluid in the intestine of neonatal but not weaned piglets.
STb is not associated with human diseases. STb unlike STa is not methanol soluble. The exact mode of action of STb is not known. The toxin causes accumulation of fluid in ligated intesti-nal loops of young piglets up to 9 weeks’ old but not in infant mice.
Heat-labile toxin:Heat-labile toxin (LT) is a heat-labile protein.The toxin was first demonstrated by De and colleagues in 1956 in E. coli isolated from cases of adult diarrhea in adults in Kolkata. They demonstrated the toxin in the bacteria by the rabbit ileal loop method, the method used for detection of the cholera enterotoxin. LT is of two types: LT-I and LT-II. LT-I but not LT-II is associated with human diseases. LT-I is structurally and antigenically similar to cholera toxin. The LT-I toxin consists of one A subunit of molecular weight of 25,000 Da and five identical B subunits, each subunit measur-ing 11,500 Da. The B subunits bind to the GM1 gangliosides, same receptor as cholera toxin, as well as other surface glyco-proteins on epithelial cells in the small intestine. This binding facilitates entry of subunit A into the cell by endocytosis. The A subunit has ADP (adenosine diphosphate)-ribosyl transfer-ase activity by which it interacts with a membrane protein (Gs) that regulates adenylate cyclase. This results in an increase in cyclic adenosine monophosphate (cAMP) levels leading to an increased secretion of chloride and a decreased absorption of sodium and chloride. This ends in watery diarrhea due to hypersecretion of fluid into the lumen of the gut. The toxin also stimulates secretion of prostaglandin and production of inflammatory cytokines, resulting in further fluid loss. Genes for LT-I and STa are present on I transferable plasmid, which can also carry the genes for adhesins (CFA/I, CFA/II, CFA/III).
◗ Pathogenesis of E. coli infections
Most infections, such as UTIs and sepsis, are endogenous and are caused by the E. coli present in large numbers in the gastro-intestinal tract of the same host. Other E. coli infections, such as gastroenteritis and neonatal meningitis, are caused by exog-enous infections, i.e., acquired from outside.
Urinary tract infections: E. coliserotypes that are normallyfound in the feces are commonly responsible for urinary tract infections. UTI is an ascending infection in which the bacteria that originate from the intestinal tract contaminate the urethra, ascend into the bladder, and may spread to the kidney or prostate.
Nephritogenic strains: Although most strains ofE. colicancause UTI, disease is more common with certain specific E.coli serogroups. These serogroups that cause UTI are knownas nephritogenic strains, these include E. coli serotypes O1, O2, O4, O6, O7, O18, etc. These serotypes cause UTI, particularly because of their ability to produce adhesins (primarily P pili, AAF/I, AAF/III, and Dr), which bind to cells lining the blad-der and upper urinary tract. This prevents elimination of the bacteria in voided urine. Only one serotype is usually isolated from urine at a time, though recurrences may be due to differ-ent serotypes. They also produce hemolysin HlyA, which lyses erythrocytes and also other cells, leading to release of cytokines and stimulation of an inflammatory response.
Gastroenteritis: Gastroenteritis is caused by exogenous infec-tions acquired from water, food, or vegetables contaminated with fecalE. coli. The strains of E. coli that cause gastroen-teritis are classified into the following six groups: (a) entero-pathogenic E. coli(EPEC), (b) enterotoxigenic E. coli (ETEC), (c) enteroinvasive E. coli (EIEC), (d) enterohemorrhagic E. coli (EHEC), (e) enteroaggregative E. coli (EAEC), and (f) diffusely adherent E. coli (DAEC) (Table 31-6).
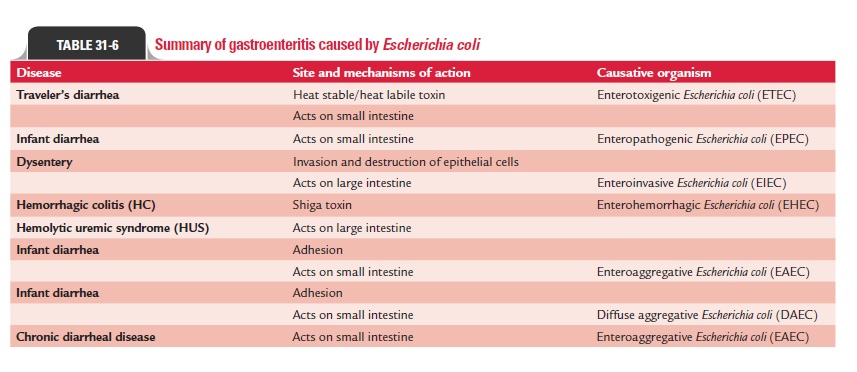
Enteropathogenic E. coli: EPEC is the major cause of infantdiarrhea in tropical countries. Disease is rare in older children and adults. EPEC strains include O26, O55, O86, O111, O114, O119, O125, O126, O12, O128, and O142. EPEC causes infec-tion by adhering to epithelial cells of the small intestine fol-lowed by destruction of the microvillus. The bacteria initially form microcolonies on the epithelial cell surface, in which the bacteria are attached to the host cells with help of cup-like pedestals. This attachment is facilitated by Bfp. This is fol-lowed by secretion of proteins by the bacterial type III secre-tion system into the host epithelial cell. Translocated intimin receptor is inserted into the epithelial cell membrane and serves as a receptor for intimin, an outer membrane bacterial adhesin of E. coli. Subsequently, the attached bacteria multiply and cause microvilli destruction, resulting in diarrhea due to malabsorption.
Enterotoxigenic E. coli: Diarrhea caused by ETEC is endemicin the developing countries, among all age groups of the pop-ulation. This is also responsible for causing traveler’s diar-rhea in which individuals from developed countries visiting endemic areas often suffer from ETEC diarrhea. The disease is caused by consumption of fecally contaminated food or water. Person-to-person spread does not occur. Although plas-mids with enterotoxin genes may be present in any strain of E. coli, diarrhea is caused by certain specific ETEC serogroups(O6, O8, O15, O25, O27, O167). These serotypes cause diar-rhea because of their ability to produce heat-labile enterotox-ins (LT-I, LT-II). LT-I, which is structurally similar to cholera toxin, produces cholera-like diarrhea in patients. The dis-ease process is facilitated further by the presence of adhesins (primarily P pili, AAF/I, AAF/III, and Dr), which bind to intes-tinal mucosa.
Enteroinvasive E. coli: EIEC strains closely resemble shigel-lae in many ways: (a) EIEC strains are nonmotile, (b) they do not ferment lactose or ferment late with production of acid only, and (c) they do not decarboxylate lysine decarboxylase. These strains show cross-reactivity with O antigen of shigel-lae. These “atypical” E. coli strains were named earlier Shigellaalkalescens under the “Alkalescens-Dispar Group” (resembling Shigella flexneri except in fermenting dulcitol and forming alkaliin litmus milk) andShigella dispar (late lactose fermenter like Shigella sonnei but indole positive). Currently, these have beenrenamed EIEC because they have the capacity to invade inter-stitial epithelial cells and also penetrate HeLa cells in tissue culture. The EIEC strains have the ability to invade and destroy the colonic epithelium, producing a disease characterized ini-tially by watery diarrhea. This ability of E. coli to invade cells is determined by a large plasmid, which codes for outer mem-brane antigens called the “virulence marker antigens” (VMA). The bacteria lyse the phagocytes and multiply in the cell cytoplasm. This continuous process of epithelial cell destruction with inflammatory infiltration leads to the development of ulcers in intestine. Specific serogroups commonly associated with out-breaks of EIEC include O28 ac, O112 ac, O124, O136, O143, O114, O152, and O154.
Enterohemorrhagic E. coli: EHEC strains are the most commoncause of gastrointestinal infections in the developed countries. These strains produce diarrheal disease, ranging in severity from mild uncomplicated diarrhea to fatal hemorrhagic coli-tis. Hemolytic uremic syndrome is a serious life-threatening complication in 10% of infected children below 10 years. The ingestion of as few as 100 bacilli can cause the disease. EHEC disease is most common in children below 5 years and in sum-mer months. The condition occurs as a result of ingestion of water, unpasteurized milk or fruit juices, ncooked vegetables, and fruits contaminated with human or animal feces. The dis-ease also occurs on consumption of undercooked ground beef or other meat products.
Serotypes O157:H7 and O26:H1 are the EHEC strains that commonly cause the disease. These strains produce Shiga tox-ins (i.e., Stx-l, Stx-2, or both), which are primarily responsible for the diarrheal diseases. Stx-2 is most commonly associated with HUS, a disorder characterized by acute renal failure, thrombocytopenia, and microangiopathic hemolytic anemia. Stx-2 causes destruction of glomerular endothelial cells, result-ing in reduced glomerular filtration and acute renal failure. The toxins also stimulate production of tumor necrosis factor-and interleukin-6, which contribute further to the disease process.
Enteroaggregative E. coli: EAEC strains are so called becausethey show a typical “stacked brick” arrangement on Hep-2 cells or glass due to their autoagglutination. Bundle-forming fimbriae of the bacteria (such as AAF/I and AAF/II), which are carried on a plasmid, mediate this process. These EAEC strains secrete a low-molecular-weight, heat-stable enterotoxin called enteroag-gregative heat-stable enterotoxin-1 (EAST-1). EAEC increases mucus secretion, which forms a layer overlying the epithelium of the small intestine. This layer of biofilm traps the bacteria in epithelium of the small intestine. In animal experiments, they cause shortening of the microvilli, mononuclear infiltration, and hemorrhage. These strains are associated with persistent, watery diarrhea with dehydration in infants, especially in devel-oping countries.
Diffusely adherent E. coli: DAEC strains cause watery diarrheafound primarily in children between 1 and 5 years of age. These strains are identified by their ability to adhere to cultured cells. They cause elongation of the microvilli with the bacteria trapped in the cell membrane.
Septicemia: Invasion of blood stream byE. colimay lead to sep-ticemia. Septicemia is caused by E. coli strains associated with UTIs or intra-abdominal infections, such as peritonitis and abscesses following intestinal perforation. The mortality due to E. colisepticemia is high for patients with immunocompro-mised status, or for patients in whom the primary infection is in the abdomen or central nervous system (CNS).
Neonatal meningitis: E. colialong with group B streptococciare the major causes of infection of the CNS in infants of age 1 month. The disease is caused by E. coli strains that pos-sess the KI capsular antigen, which are commonly present in the gastrointestinal tracts of pregnant women and newborn infants.
◗ Host immunity
Disease caused by EPEC is rare in older children and adults, presumably because they have developed protective immunity. Immunity develops to ETEC surface antigens in local adult populations; hence disease is confined to immunologically naïve travelers and weaning infants.
Related Topics