Chapter: Basic & Clinical Pharmacology : Drugs Used in Disorders of Coagulation
Oral Direct Factor XA Inhibitors
ORAL DIRECT FACTOR XA INHIBITORS
Oral Xa inhibitors,
including rivaroxaban and apixaban, are approved or in advanced stages of development
and along with oral thrombin inhibitors (discussed below) are likely to have a
major impact on antithrombotic pharmacotherapy. These drugs inhibit factor Xa,
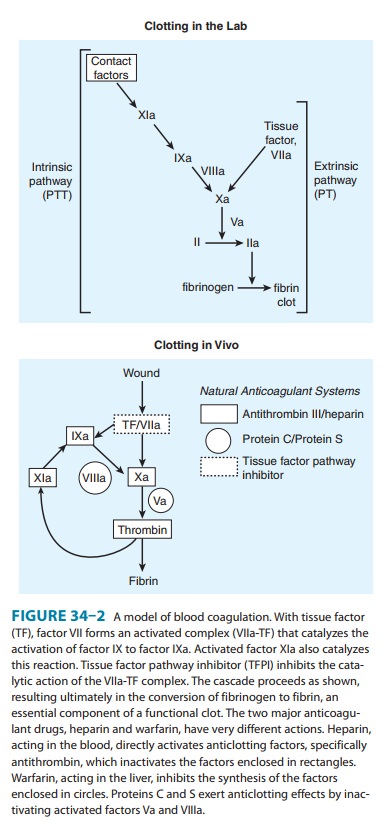
in the final common pathway of clotting (see Figure 34–2). These drugs are
given as fixed doses and do not require monitoring. They have a rapid onset of
action and shorter half-lives than warfarin (approximately 10 hours but
half-life may be prolonged in elderly patients or those with renal impairment).
Rivaroxaban is approved for prevention of venous throm-boembolism following
hip or knee surgery. The prophylactic dose is 10 mg orally per day. A recent
large randomized clinical trial of DVT treatment compared a higher dose of
rivaroxaban (15 mg bid for 3 weeks, followed by 20 mg daily) to a standard
treatment regimen of enoxaparin followed by warfarin. This trial demon-strated
non-inferiority of rivaroxaban in preventing recurrent venous thromboembolism
and showed no difference in bleeding risk. Another trial reported
non-inferiority of rivaroxaban to war-farin for prevention of stroke in
patients with atrial fibrillation.
Apixaban is currently in clinical development. A recent studyof patients
undergoing total hip replacement compared apixaban 2.5 mg orally once per day
with enoxaparin 40 mg subcutane-ously once per day. This trial demonstrated the
apixaban arm had lower rates of venous thromboembolism and similar
bleedingrates. Another multicenter study randomized patients with recent
myocardial infarction to apixaban 5 mg or placebo. This trial was stopped early
because of an increase in bleeding risk without a significant decrease in
ischemic events. Another trial comparing apixaban to aspirin for stroke
prevention in atrial fibrillation was stopped early because of evidence of
increased efficacy in the apixaban arm.
Thus, it appears that
the primary target populations for devel-opment of both rivaroxaban and
apixaban will be prevention and treatment of patients with venous
thromboembolism and stroke prevention in patients with atrial fibrillation.
Both of these drugs are excreted in part by the kidneys; therefore, the dosage
may need to be reduced in patients with renal impairment. In such patients, use
of a hepatically metabolized drug such as warfarin may be a better alternative.
No antidotes exist for direct Xa inhibitors.
Related Topics