Chapter: Basic & Clinical Pharmacology : Drugs Used in Disorders of Coagulation
Basic Pharmacology of Antiplatelet Agents
BASIC PHARMACOLOGY OF ANTIPLATELET AGENTS
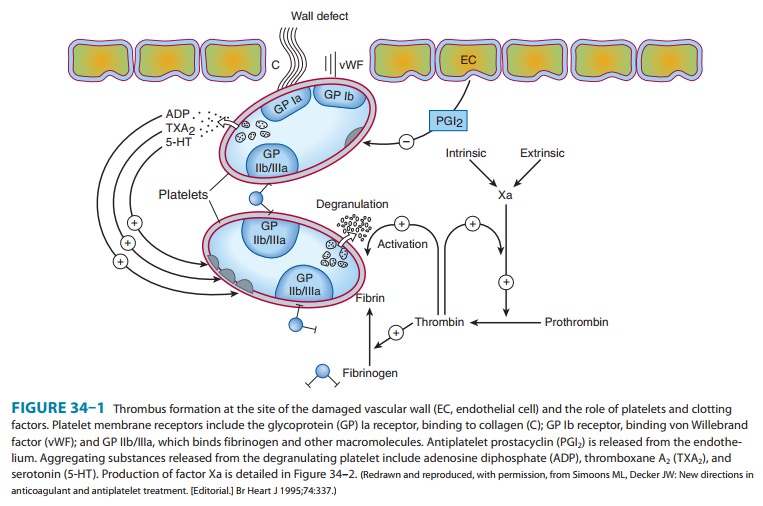
Platelet function is
regulated by three categories of substances. The first group consists of agents
generated outside the platelet that interact with platelet membrane receptors,
eg, catecholamines, collagen, thrombin, and prostacyclin. The second category
con-tains agents generated within the platelet that interact with mem-brane
receptors, eg, ADP, prostaglandin D2, prostaglandin E2, and serotonin. The third group comprises
agents generated within the platelet that act within the platelet, eg, prostaglandin
endoperox-ides and thromboxane A2, the cyclic nucleotides cAMP and cGMP, and calcium ion. From
this list of agents, several targets for platelet inhibitory drugs have been
identified (Figure 34–1): inhi-bition of prostaglandin synthesis (aspirin), inhibition
of ADP-induced platelet aggregation (clopidogrel, prasugrel, ticlopidine), and
blockade of glycoprotein IIb/IIIa receptors on platelets (abcix-imab,
tirofiban, and eptifibatide). Dipyridamole and cilostazol are additional
antiplatelet drugs.
ASPIRIN
The prostaglandin thromboxane A2 is an arachidonate product that causes platelets to change
shape, release their granules, and aggregate . Drugs that antagonize this
pathway interfere with platelet aggregation in vitro and prolong the bleed-ing
time in vivo. Aspirin is the prototype of this class of drugs.
As described, aspirin
inhibits the synthesis of thromboxane A2 by irreversible acetylation of the enzyme
cycloox-ygenase. Other salicylates and nonsteroidal anti-inflammatory drugs
also inhibit cyclooxygenase but have a shorter duration of inhibitory action
because they cannot acetylate cyclooxygenase; that is, their action is
reversible.
The FDA has approved
the use of 325 mg/d aspirin for primary
prophylaxis of myocardial infarction but urges caution in this use of aspirin
by the general population except when prescribed as an adjunct to risk factor
management by smoking cessation and low-ering of blood cholesterol and blood
pressure. Meta-analysis of many published trials of aspirin and other antiplatelet
agents con-firms the value of this intervention in the secondary prevention of vascular events among patients with a
history of vascular events.
TICLOPIDINE, CLOPIDOGREL, & PRASUGREL
Ticlopidine,
clopidogrel, and prasugrel reduce platelet aggrega-tion by inhibiting the ADP
pathway of platelets. These drugs irreversibly block the ADP receptor on
platelets. Unlike aspirin, these drugs have no effect on prostaglandin
metabolism. Use of ticlopidine, clopidogrel, or prasugrel to prevent thrombosis
is now considered standard practice in patients undergoing place-ment of a
coronary stent. As the indications and adverse effects of these drugs are
different, they will be considered individually.
Ticlopidine is
approved for prevention of stroke in patients with a history of a transient
ischemic attack (TIA) or thrombotic stroke, and in combination with aspirin for
prevention of coro-nary stent thrombosis. Adverse effects of ticlopidine
include nau-sea, dyspepsia, and diarrhea in up to 20% of patients, hemorrhage
in 5%, and, most seriously, leukopenia in 1%. The leukopenia is detected by
regular monitoring of the white blood cell count dur-ing the first 3 months of
treatment. Development of thrombotic thrombocytopenic purpura has also been
associated with the ingestion of ticlopidine. The dosage of ticlopidine is 250
mg twice daily. Because of the significant side effect profile, the use of
ticlo-pidine for stroke prevention should be restricted to those who are
intolerant of or have failed aspirin therapy. Doses of ticlopidine less than
500 mg/d may be efficacious with fewer adverse effects.
Clopidogrel is
approved for patients with unstable angina or non-ST-elevation acute myocardial
infarction (NSTEMI) in com-bination with aspirin; for patients with
ST-elevation myocardial infarction (STEMI); or recent myocardial infarction,
stroke, or established peripheral arterial disease. For NSTEMI, the dosage is a
300 mg loading dose followed by 75 mg daily of clopidogrel, with a daily
aspirin dose of 75–325 mg. For patients with STEMI, the dose is 75 mg daily of
clopidogrel, in association with aspirin as above; and for recent myocardial
infarction, stroke, or periph-eral vascular disease, the dose is 75 mg/d.
Clopidogrel has fewer
adverse effects than ticlopidine and is rarely associated with neutropenia.
Thrombotic thrombocytopenic purpura has been reported. Because of its superior
side effect pro-file and dosing requirements, clopidogrel is frequently
preferred over ticlopidine. The antithrombotic effects of clopidogrel are
dose-dependent; within 5 hours after an oral loading dose of 300 mg, 80% of
platelet activity will be inhibited. The mainte-nance dose of clopidogrel is 75
mg/d, which achieves maximum platelet inhibition. The duration of the
antiplatelet effect is 7–10 days. Clopidogrel is a prodrug that requires
activation via the cytochrome P450 enzyme isoform CYP2C19. Depending on the
single nucleotide polymorphism inheritance pattern in CYP2C19, individuals may
be poor metabolizers of clopidogrel, and these patients may be at increased
risk of cardiovascular events due to inadequate drug effect. The FDA has
recommended CYP2C19 genotyping to identify such patients and advises
prescribers to consider alternative therapies in poor metabolizers. However,
more recent studies have questioned the impact of CYP2C19 metabolizer status on
outcomes. Drugs that impair CYP2C19 function, such as omeprazole, should be
used with cau-tion pending clarification of the importance of CYP2C19 status.
Prasugrel, similar to
clopidogrel, is approved for patients with acute coronary syndromes. The drug
is given as a 60-mg loading dose and then 10 mg/d in combination with aspirin
as outlined for clopidogrel. The Trial to assess Improvement in Therapeutic
Outcomes by Optimizing Platelet Inhibition with Prasugrel (TRITON-TIMI38)
compared prasugrel with clopidogrel in a randomized, double-blind trial with
aspirin and other standard therapies managed with percutaneous coronary
interventions. This trial showed a reduction in the primary composite cardio-vascular
endpoint (cardiovascular death, nonfatal stroke or non-fatal myocardial
infarction) for prasugrel in comparison with clopidogrel. However, the major
and minor bleeding risk was increased with prasugrel. Prasugrel is
contraindicated in patients with history of TIA or stroke because of increased
bleeding risk. Although cytochrome P450 genotype status is an issue with
clopidogrel, it does not have an impact on the use of prasugrel.
Aspirin & Clopidogrel Resistance
The reported incidence
of resistance to these drugs varies greatly, from less than 5% to 75%. In part
this tremendous variation in incidence reflects the definition of resistance
(recurrent thrombo-sis while on antiplatelet therapy vs in vitro testing),
methods by which drug response is measured, and patient compliance. Several
methods for testing aspirin and clopidogrel resistance in vitro are now
FDA-approved. However, the incidence of drug resistance varies considerably by
testing method. These tests may be useful in selected patients to assess
compliance or the cause of a recurrent thrombotic event, but their utility in
routine clinical decision making outside of clinical trials remains
controversial.
BLOCKADE OF PLATELET GLYCOPROTEIN IIB/IIIA RECEPTORS
The glycoprotein
IIb/IIIa inhibitors are used in patients with acute coronary syndromes. These
drugs target the platelet IIb/IIIa receptor complex (Figure 34–1). The IIb/IIIa
complex functions as a receptor mainly for fibrinogen and vitronectin but also
for fibronectin and von Willebrand factor. Activation of this receptor complex
is the “final common pathway” for platelet aggregation. There are approximately
50,000 copies of this complex on the surface of each platelet. Persons lacking
this receptor have a bleed-ing disorder called Glanzmann’s thrombasthenia.
Abciximab, a chimeric monoclonal antibody directed againstthe IIb/IIIa
complex including the vitronectin receptor, was the first agent approved in
this class of drugs. It has been approved for use in percutaneous coronary
intervention and in acute coronary syndromes. Eptifibatide is an analog of the sequence at the extreme carboxyl
terminal of the delta chain of fibrinogen, which mediates the binding of
fibrinogen to the receptor. Tirofiban
is a smaller molecule with similar properties. Eptifibatide and tiro-fiban
inhibit ligand binding to the IIb/IIIa receptor by their occu-pancy of the
receptor but do not block the vitronectin receptor.
The three agents
described above are administered parenterally. Oral formulations of IIb/IIIa
antagonists are in various stages of development.
ADDITIONAL ANTIPLATELET-DIRECTED DRUGS
Dipyridamole is a vasodilator that also inhibits platelet functionby
inhibiting adenosine uptake and cGMP phosphodiesterase activity. Dipyridamole
by itself has little or no beneficial effect. Therefore, therapeutic use of
this agent is primarily in combina-tion with aspirin to prevent cerebrovascular
ischemia. It may also be used in combination with warfarin for primary
prophylaxis ofthromboemboli in patients with prosthetic heart valves. A
combi-nation of dipyridamole complexed with 25 mg of aspirin is now available
for secondary prophylaxis of cerebrovascular disease.
Cilostazol is a newer phosphodiesterase inhibitor that pro-motes
vasodilation and inhibition of platelet aggregation. Cilostazol is used
primarily to treat intermittent claudication.
Related Topics