Chapter: Basic & Clinical Pharmacology : Drugs Used in Disorders of Coagulation
Basic Pharmacology of the Fibrinolytic Drugs
BASIC PHARMACOLOGY OF THE FIBRINOLYTIC DRUGS
Fibrinolytic drugs
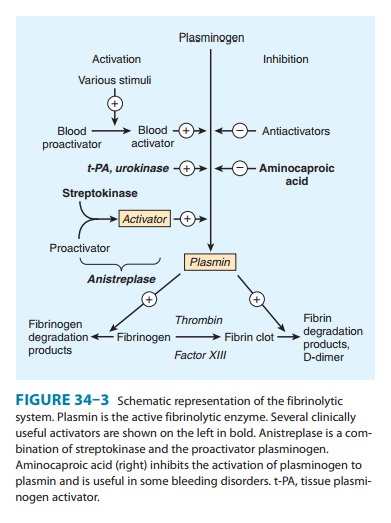
rapidly lyse thrombi by catalyzing the for-mation of the serine protease plasmin from its precursor zymo-gen,
plasminogen (Figure 34–3). These drugs create a generalized lytic state when
administered intravenously. Thus, both protective hemostatic thrombi and target
thromboemboli are broken down. The Box: Thrombolytic Drugs for Acute Myocardial
Infarction describes the use of these drugs in one major application.
Pharmacology
Streptokinase is
a protein (but not an enzyme in itself ) synthe-sized by streptococci that
combines with the proactivator plasmi-nogen. This enzymatic complex catalyzes
the conversion of inactive plasminogen to active plasmin. Urokinase is a human enzyme synthesized by the kidney that directly
converts plasmi-nogen to active plasmin. Plasmin itself cannot be used because
naturally occurring inhibitors in plasma prevent its effects. However, the
absence of inhibitors for urokinase and the strepto-kinase-proactivator complex
permits their use clinically. Plasmin formed inside a thrombus by these
activators is pro-tected from plasma antiplasmins, which allows it to lyse the
thrombus from within.Anistreplase (anisoylated
plasminogen streptokinase activatorcomplex; APSAC) consists of a complex of purified
human plas-minogen and bacterial streptokinase that has been acylated to
protect the enzyme’s active site. When administered, the acyl group
spontaneously hydrolyzes, freeing the activated streptoki-nase-proactivator
complex. This product (now discontinued in the USA) allows for rapid
intravenous injection, greater clot selectivity (ie, more activity on
plasminogen associated with clots than on free plasminogen in the blood), and
more thrombolytic activity.
Plasminogen can also
be activated endogenously by tissueplasminogen
activators (t-PAs). These activators preferentiallyactivate plasminogen
that is bound to fibrin, which (in theory) confines fibrinolysis to the formed
thrombus and avoids systemic activation. Human t-PA is manufactured as alteplase by means of recombinant DNA
technology. Reteplase is another
recombinant human t-PA from which several amino acid sequences have been
deleted. Reteplase is less expensive to produce than t-PA. Because it lacks the
major fibrin-binding domain, reteplase is less fibrin-specific than t-PA. Tenecteplase is a mutant form of t-PA
that has a longer half-life, and it can be given as an intravenous bolus.
Tenecteplase is slightly more fibrin-specific than t-PA.
Indications & Dosage
Administration
of fibrinolytic drugs by the intravenous route is indicated in cases of pulmonary embolism with
hemodynamicinstability, severe deep
venous thrombosis such as the superiorvena caval syndrome, and ascending thrombophlebitis of the
iliofemoral vein with severe lower extremity edema. These drugs are also given
intra-arterially, especially for peripheral vascular disease.
Thrombolytic
therapy in the management of acute
myocar-dial infarction requires careful patient selection, the use of
aspecific thrombolytic agent, and the benefit of adjuvant therapy.
Streptokinase is administered by intravenous infusion of a load-ing dose of
250,000 units, followed by 100,000 units/h for 24–72 hours. Patients with
antistreptococcal antibodies can develop fever, allergic reactions, and therapeutic
resistance. Urokinase requires a loading dose of 300,000 units given over 10
minutes and a maintenance dose of 300,000 units/h for 12 hours. Alteplase
(t-PA) is given by intravenous infusion of 60 mg over the first hour and then
40 mg at a rate of 20 mg/h. Reteplase is given as two intravenous bolus
injections of 10 units each, sepa-rated by 30 minutes. Tenecteplase is given as
a single intravenous bolus of 0.5 mg/kg. Anistreplase (where available) is
given as a single intravenous injection of 30 units over 3–5 minutes. A sin-gle
course of fibrinolytic drugs is expensive: hundreds of dollars for
streptokinase and thousands for urokinase and t-PA.
Recombinant
t-PA has also been approved for use in acute isch-emic stroke within 3 hours of
symptom onset. In patients without hemorrhagic infarct or other
contraindications, this therapy has been demonstrated to provide better
outcomes in several random-ized clinical trials. The recommended dose is 0.9
mg/kg, not to exceed 90 mg, with 10% given as a bolus and the remainder during
a 1 hour infusion. Streptokinase has been associated with increased bleeding
risk in acute ischemic stroke when given at a dose of 1.5 million units, and
its use is not recommended in this setting.
Related Topics