Chapter: Basic & Clinical Pharmacology : Drugs Used in Disorders of Coagulation
Indirect Thrombin Inhibitors
INDIRECT THROMBIN INHIBITORS
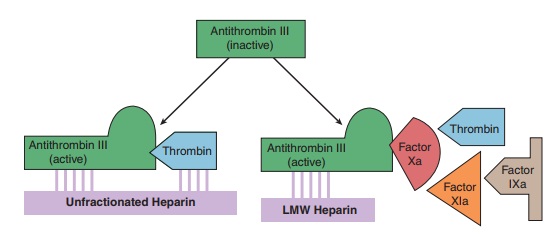
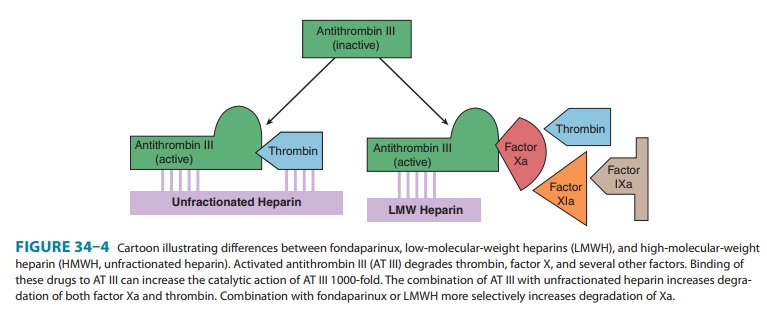
The indirect thrombin inhibitors are so-named because their anti-thrombotic effect is exerted by their interaction with a separate protein, antithrombin. Unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), and the synthetic penta-saccharide fondaparinux bind to antithrombin and enhance its inactivation of factor Xa (Figure 34–4). Unfractionated heparin and to a lesser extent LMWH also enhance antithrombin’s inacti-vation of thrombin.
HEPARIN
Chemistry & Mechanism of Action
Heparin is a heterogeneous mixture of sulfated mucopolysaccha-rides. It binds to endothelial cell surfaces and a variety of plasma proteins. Its biologic activity is dependent upon the endogenous anticoagulant antithrombin. Antithrombin inhibits clotting fac-tor proteases, especially thrombin (IIa), IXa, and Xa, by forming equimolar stable complexes with them. In the absence of heparin, these reactions are slow; in the presence of heparin, they are accelerated 1000-fold.
Only about a third of the molecules in commercial heparin preparations have an accelerating
effect because the remainder lack the unique pentasaccharide sequence needed
for high-affinity binding to antithrombin. The active heparin molecules bind
tightly to antithrombin and cause a con-formational change in this inhibitor.
The conformational change of antithrombin exposes its active site for more
rapid interaction with the proteases (the activated clotting factors). Heparin
func-tions as a cofactor for the antithrombin-protease reaction without being
consumed. Once the antithrombin-protease complex is formed, heparin is released
intact for renewed binding to more antithrombin.
The antithrombin binding region of commercial
unfraction-ated heparin consists of repeating sulfated disaccharide units
composed of D-glucosamine-L-iduronic acid and D-glucosamine-D-glucuronic acid.
High-molecular-weight (HMW), also knownas UFH, fractions of heparin with high
affinity for antithrombin markedly inhibit blood coagulation by inhibiting all
three factors, especially thrombin and factor Xa. Unfractionated heparin has a
molecular weight range of 5000–30,000. In contrast, the shorter-chain
low-molecular-weight (LMW) fractions of heparin inhibit activated factor X but
have less effect on thrombin than the HMW species. Nevertheless, numerous
studies have demonstrated that LMW heparins such as enoxaparin, dalteparin, and tinzaparin
are effective in several thromboembolic conditions. In fact, these LMW
heparins—in comparison with UFH—have equal efficacy, increased bioavailability
from the subcutaneous site of injection, and less frequent dosing requirements
(once or twice daily is sufficient).
Because
commercial heparin consists of a family of molecules of different molecular
weights extracted from porcine intestinal mucosa and bovine lung, the
correlation between the concentra-tion of a given heparin preparation and its
effect on coagulation often is poor. Therefore, UFH is standardized by
bioassay. Heparin was reformulated in 2009 in response to heparin
con-tamination events in 2007 and 2008. The contaminant was iden-tified as
over-sulfated chondroitin sulfate and linked to more than150 adverse events in
patients, most commonly hypotension, nausea, and dyspnea within 30 minutes of
infusion. In response to this event, heparin sodium was reformulated with
stricter qual-ity control measures and bioassays to make detection of
contami-nants easier. This reformulation led to a decrease in potency of
approximately 10% from the previous formulation. USP heparin is now harmonized
to the World Health Organization International Standard (IS) unit dose.
Enoxaparin is obtained from the same sources as regular unfractionated heparin,
but doses are specified in milligrams. Dalteparin, tinzaparin, and danaparoid
(an LMW heparanoid containing heparan sulfate, dermatan sulfate, and
chondroitin sulfate), on the other hand, are specified in anti-factor Xa units.
Monitoring of Heparin Effect
Close
monitoring of the activated partial
thromboplastin time(aPTT or PTT) is
necessary in patients receiving UFH. Levels ofUFH may also be determined by
protamine titration (therapeu-tic levels 0.2–0.4 unit/mL) or anti-Xa units
(therapeutic levels 0.3–0.7 unit/mL). Weight-based dosing of the LMW heparins
results in predictable pharmacokinetics and plasma levels in patients with
normal renal function. Therefore, LMW heparin levels are not generally measured
except in the setting of renal insufficiency, obesity, and pregnancy. LMW
heparin levels can be determined by anti-Xa units. Peak therapeutic levels
should be 0.5–1 unit/mL for twice-daily dosing, determined 4 hours after
administration, and approximately 1.5 units/mL for once-daily dosing.
Toxicity
A. Bleeding and Miscellaneous Effects
The major adverse
effect of heparin is bleeding. This risk can be decreased by scrupulous patient
selection, careful control of dos-age, and close monitoring. Elderly women and
patients with renal failure are more prone to hemorrhage. Heparin is of animal
origin and should be used cautiously in patients with allergy. Increased loss
of hair and reversible alopecia have been reported. Long-term heparin therapy
is associated with osteoporosis and spontaneous fractures. Heparin accelerates
the clearing of postprandial lipemia by causing the release of lipoprotein
lipase from tissues, and long-term use is associated with mineralocorticoid
deficiency.
B. Heparin-Induced Thrombocytopenia
Heparin-induced
thrombocytopenia (HIT) is a systemic hyperco-agulable state that occurs in 1–4%
of individuals treated with UFH for a minimum of 7 days. Surgical patients are
at greatest risk. The reported incidence of HIT is lower in pediatric
popula-tions outside the critical care setting and is relatively rare in
preg-nant women. The risk of HIT may be higher in individuals treated with UFH
of bovine origin compared with porcine heparin and is lower in those treated
exclusively with LMWH.
Morbidity
and mortality in HIT are related to thrombotic events. Venous thrombosis occurs
most commonly, but occlusion of peripheral or central arteries is not
infrequent. If an indwelling catheter is present, the risk of thrombosis is
increased in that extremity. Skin necrosis has been described, particularly in
individu-als treated with warfarin in the absence of a direct thrombin
inhibi-tor, presumably due to acute depletion of the vitamin K-dependent anticoagulant
protein C occurring in the presence of high levels of procoagulant proteins and
an active hypercoagulable state.
The
following points should be considered in all patients receiving heparin:
Platelet counts should be performed frequently; thrombocytopenia appearing in a
time frame consistent with an immune response to heparin should be considered
suspicious for HIT; and any new thrombus occurring in a patient receiving
heparin therapy should raise suspicion of HIT. Patients who develop HIT are treated
by discontinuance of heparin and admin-istration of a direct thrombin
inhibitor.
Contraindications
Heparin
is contraindicated in patients with HIT, hypersensitivity to the drug, active
bleeding, hemophilia, significant thrombocy-topenia, purpura, severe
hypertension, intracranial hemorrhage, infective endocarditis, active
tuberculosis, ulcerative lesions of the gastrointestinal tract, threatened
abortion, visceral carcinoma, or advanced hepatic or renal disease. Heparin
should be avoided in patients who have recently had surgery of the brain,
spinal cord, or eye, and in patients who are undergoing lumbar puncture or
regional anesthetic block. Despite the apparent lack of placental transfer,
heparin should be used in pregnant women only when clearly indicated.
Administration & Dosage
The indications for
the use of heparin are described in the section on clinical pharmacology. A
plasma concentration of heparin of 0.2–0.4 unit/mL (by protamine titration) or
0.3–0.7 unit/mL (anti-Xa units) usually prevents pulmonary emboli in patients
with established venous thrombosis. This concentration generally corresponds to
a PTT of 2–3 times baseline. However, the use of the PTT for heparin monitoring
is problematic. There is no stan-dardization scheme for the PTT as there is for
the prothrombin time (PT) and its international normalized ratio (INR).
Currentlymore than 300 reagent-instrument combinations are in use, and the
actual ratios required to obtain an anti-Xa activity of 0.3–0.7 units/mL are
variable, ranging from 1.6 to 6 times control PTT. Thus, if the PTT is used for
monitoring, the laboratory should determine the clotting time that corresponds
to the thera-peutic range by protamine titration or anti-Xa activity, as listed
above.
In addition, some
patients have a prolonged baseline PTT due to factor deficiency or inhibitors
(which could increase bleeding risk) or lupus anticoagulant (which is not
associated with bleeding risk but may be associated with thrombosis risk).
Using the PTT to assess heparin effect in such patients is very difficult. An
alter-native is to use anti-Xa activity to assess heparin concentration, a test
now widely available on automated coagulation instruments. This approach more
accurately measures the heparin concentra-tion; however, it does not provide
the global assessment of intrin-sic pathway integrity of the PTT.
The following strategy
is recommended: prior to initiating anticoagulant therapy of any type, the
integrity of the patient’s hemostatic system should be assessed by a careful
history of prior bleeding events, and baseline PT and PTT. If there is a
prolonged clotting time, the cause of this (deficiency or inhibitor) should be
determined prior to initiating therapy, and treatment goals strati-fied to a
risk-benefit assessment. In high-risk patients measuring both the PTT and
anti-Xa activity may be useful. When intermit-tent
heparin administration is used, the aPTT or anti-Xa activityshould be
measured 6 hours after the administered dose to main-tain prolongation of the
aPTT to 2–2.5 times that of the control value. However, LMW heparin therapy is
the preferred option in this case, as no monitoring is required in most
patients.
Continuous intravenous
administration of heparin is accom-plished via an infusion pump. After an
initial bolus injection of 80–100 units/kg, a continuous infusion of about
15–22 units/ kg/h is required to maintain the anti-Xa activity in the range of
0.3–0.7 units/mL. Low-dose prophylaxis is achieved with subcu-taneous
administration of heparin, 5000 units every 8–12 hours. Because of the danger
of hematoma formation at the injection site, heparin must never be administered
intramuscularly.
Prophylactic
enoxaparin is given subcutaneously in a dosage of 30 mg twice daily or 40 mg
once daily. Full-dose enoxaparin therapy is 1 mg/kg subcutaneously every 12
hours. This corre-sponds to a therapeutic anti-factor Xa level of 0.5–1
unit/mL. Selected patients may be treated with enoxaparin 1.5 mg/kg once a day,
with a target anti-Xa level of 1.5 units/mL. The prophylac-tic dose of
dalteparin is 5000 units subcutaneously once a day; therapeutic dosing is 200
units/kg once a day for venous disease or 120 units/kg every 12 hours for acute
coronary syndrome. LMWH should be used with caution in patients with renal
insuf-ficiency or body weight greater than 150 kg. Measurement of the anti-Xa
level is useful to guide dosing in these individuals.
The synthetic
pentasaccharide molecule fondaparinux
(Figure 34–4) avidly binds antithrombin with high specific activ-ity, resulting
in efficient inactivation of factor Xa. Fondaparinux has a long half-life of 15
hours, allowing for once-daily dosing by subcutaneous administration.
Fondaparinux is effective in the prevention and treatment of venous
thromboembolism, and appears to not cross-react with pathologic HIT antibodies
in most individuals. The use of fondaparinux as an alternative anticoagu-lant
in HIT is currently being tested in clinical trials.
A major focus of drug
development has been to develop orally active anticoagulants that do not
require monitoring. Rivaroxaban is
the first oral factor Xa inhibitor to reach phase III clinical trials. The
safety and efficacy of rivaroxaban appears to be at least equivalent, and
possibly superior, to LMW heparins .
Reversal of Heparin Action
Excessive
anticoagulant action of heparin is treated by discontinu-ance of the drug. If
bleeding occurs, administration of a specific antagonist such as protamine sulfate is indicated.
Protamine is a highly basic, positively charged peptide that combines with
negatively charged heparin as an ion pair to form a stable complex devoid of
anticoagulant activity. For every 100 units of heparin remaining in the
patient, 1 mg of protamine sulfate is given intra-venously; the rate of
infusion should not exceed 50 mg in any 10-minute period. Excess protamine must
be avoided; it also has an anticoagulant effect. Neutralization of LMW heparin
by protamine is incomplete. Limited experience suggests that 1 mg of protamine
sulfate may be used to partially neutralize 1 mg of enoxaparin. Protamine will
not reverse the activity of fondaparinux. Excess dan-aparoid can be removed by
plasmapheresis.
Related Topics