Chapter: Biology of Disease: Toxicology
Metals - Toxicology Poisons
METALS
The presence of certain metals in high concentrations may be
toxic to humans. To diagnose metal toxicity, three features have to be
identified, namely, a source of the toxic metal, the presence of signs and
symptoms typical of toxicity by that metal and increased concentrations of the
metal in the body tissues. Metals that are commonly screened for toxicity
include lithium, aluminum and the heavy metals lead, arsenic, cadmium and
mercury. Lithium is widely used therapeutically to treat patients with certain
psychiatric disorders. However, plasma concentrations of lithium in excess of
1.5 mmol dm–3 should be avoided and regular measurements of serum
lithium concentrations are important in monitoring therapy. Lithium toxicity is
associated with tremors, drowsiness, tinnitus, blurred vision, polyuria,
hypothyroidism and, in severe cases, renal failure and coma.
Acute poisoning with aluminum is extremely rare. Indeed,
aluminum compounds are used for their antacid properties. The dietary intake of
aluminum is 5 to 10 mg day–1 and this amount is removed completely
by the kidneys. Unfortunately, patients with renal failure are susceptible to
aluminum toxicity. They cannot remove the aluminum and, as the water used in
dialysis may contain aluminum that can enter the body through the dialysis
membrane, the metal can build up to toxic concentrations and cause
osteodystrophy and encephalopathy. Aluminum toxicity is diagnosed by determining
its concentration in plasma. Chronic toxicity occurs at concentrations above
only 3 Mmol dm–3 whereas 10 Mmol dm–3 can cause acute poisoning. The treatment is
aimed mainly at prevention. When aluminum poisoning does occur, then its
excretion may be enhanced using chelating agents such as desferrioxamine.
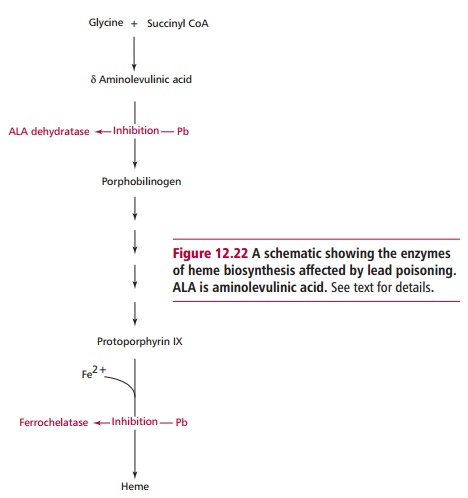
Lead is a heavy metal found in the environment. Common sources
include paints, old water pipes, petrol fumes, industrial pollution and
medication and cosmetics from the Indian subcontinent. Poisoning by lead can be
acute, which is rare, or, more commonly, chronic. It results from inhalation,
dermal absorption or ingestion, where it is actively absorbed by the GIT
through the calcium transport system. All tissues are affected by lead
poisoning although organ pathology is associated primarily with the nervous
system and the blood. In the blood, lead is concentrated in the erythrocytes
where it inhibits the activities of aminolevulinic acid (ALA) dehydratase and
ferrochelatase, enzymes involved in the synthesis of heme (Figure 12.22). Inhibition of ALA dehydratase is the most sensitive
measure of lead poisoning. Concentrations of lead in the blood in excess of
0.49 Mmol dm–3 are associated with illness in
children. If the concentration of lead increases above 3.4 Mmol dm–3,individuals
whose occupation exposes them to lead must be removed from its
source. Clinical features of lead poisoning include lethargy, abdominal
discomfort, anemia, constipation, encephalopathy and motor peripheral
neuropathy.
The management of patients with lead poisoning involves
identifying the lead source and removing the patients from it. Patients are
placed on chelating agents, such as EDTA (Figure
12.23) that is effective in both acute and chronic lead poisoning, but has
to be given intravenously. Another chelating agent, dimercaptosuccinic acid is
less efficient as a chelator but can be given orally.
Chronic arsenic poisoning is associated with well water
contaminated with arsenical pesticides or, classically, with murder! Arsenic
occurs in a number of different forms, most of which are toxic although
arsenite (AsO2–) is much more
so than arsenate (HAsO4–). Arsenate
substitutes for Pi in biological systems, hence less ATP is produced.
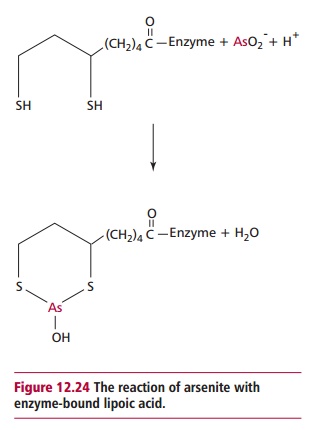
Arsenite toxicity is associated with the formation of a stable complex with
enzyme-bound lipoic acids (Figure 12.24).
Indeed, arsenic poisoning can be explained by its ability to inhibit enzymes,
such as pyruvate dehydrogenase, 2-oxoglutarate dehydrogenase and branched chain
α -oxoacid dehydrogenase, that require lipoic acid as
a coenzyme. Chronic poisoning with arsenic is usually associated with diarrhea,
polyneuropathy and dermatitis whereas acute poisoning with arsenic gives rise
to severe gastrointestinal pain, vomiting and shock. Poisoning can be diagnosed
by determining the concentration of arsenic in the hair or fingernails of the
victim. Values larger than 0.5 µg g–1 of hair indicate a significant
exposure to arsenic. The hair of a person chronically exposed to arsenic could
have 1000 times as much as this. Treatment of arsenic poisoning is aimed at
enhancing its excretion using chelating agents.
Chronic cadmium toxicity may occur in workers exposed to fumes
in cadmium-related industries, although concentrations of cadmium are twice as
high in tobacco smokers compared with nonsmokers. Concentrations in the serum
greater than 90 nmol dm–3 are associated with toxicity. Cadmium
poisoning causes influenza-like symptoms, such as chills, fever, muscular
aches, nausea, vomiting, abdominal pain, and diarrhea. However, these symptoms
may resolve after a week provided there is no respiratory damage. More severe
exposures can cause bronchitis and pulmonary edema and occasionally
cardiovascular collapse. Long-term exposure may lead to nephrotoxicity with
proteinuria, bone disease and hepatotoxicity. The treatment of cadmium poisoning
usually involves removal from exposure. Treatment with chelating agents is not
effective because the soluble form of cadmium damages the kidney.
Mercury poisoning can be acute or chronic. It occurs following
exposure to mercury vapor, inorganic salts or organic compounds of mercury.
Mercury poisoning is primarily occupational but can be caused by contaminated
food. The clinical features of acute mercury poisoning include coughing,
bronchiolitis, pulmonary edema, pneumonitis, peripheral neuropathy and
neuropsychiatric problems. Chronic mercury poisoning causes anorexia, sweating,
insomnia, impaired memory, paresthesiae of the lips and extremities and renal
tubular damage. Mercury poisoning is usually diagnosed by determining the
concentration of mercury in serum and urine. Urine mercury/creatinine ratios
are often used to assess exposure. Ratios of 40 to 100 nmol mmol–1
require monitoring and further investigation, while values of greater than 100
nmol mmol–1 require the patient to be removed from the source of
mercury.
Acute mercury poisoning is treated with chelating agents such as
dimercaprol, to increase its excretion into bile and urine. Methylmercury is an
organic form of mercury that has been used to preserve seed grain.
Methylmercury poisoning can be caused by eating meat from animals which have
been fed treated seedgrain. An outbreak of mercury poisoning began in the 1950s
in Minamata bay in Japan and affected over 3000 villagers who ate fish
contaminated with methylmercury.
Related Topics