Chapter: Biology of Disease: Toxicology
Physiological Detoxification Mechanisms
PHYSIOLOGICAL DETOXIFICATION
MECHANISMS
Xenobiotics, whether drugs or toxins, are normally
absorbed through the lungs, GIT or the skin. In addition, drugs may be injected
through intramuscular, intraperitoneal, subcutaneous or intravenous routes.
Following absorption, the xenobiotics are distributed around the body and a
proportion will be absorbed by cells, while some may be directly excreted from
the kidneys. Humans also have a number of physiological mechanisms devoted to
detoxifying ingested xenobiotics, which largely involve enzyme activities in
the liver and kidneys. Many of the biochemical detoxification mechanisms
convert the xenobiotic to more water-soluble compounds that are more easily
excreted. These mechanisms can sometimes prove inadequate. Also, in some
unfortunate cases, the detoxification mechanisms form compounds that are even
more toxic or carcinogenic than the original compound.
Treatment for poisoning involves administering an
antidote to the toxin or drug when one is available. For many common poisons
this is not the case and therapy involves general supportive measures, such as
decreasing their absorption from the GIT, increasing the rate of elimination
from the body or altering the distribution within the body to protect
susceptible tissues. An optimal therapeutic concentration of a drug can only be
maintained in the plasma if the patient fully complies with the prescribed
dose. Unfortunately, the most common reason for emergency admissions to
hospitals is because of an excessive intake of a drug prescribed for
medication. Other causes of poisoning may be accidental, suicidal or homicidal
in intent.
Many poisons are lipophilic and are only sparingly soluble in
water and so cannot easily be excreted by the kidneys. Thus a major aim of
detoxification in the liver is to convert them to compounds that have increased
water solubility and are more readily removed by the kidneys. Detoxification
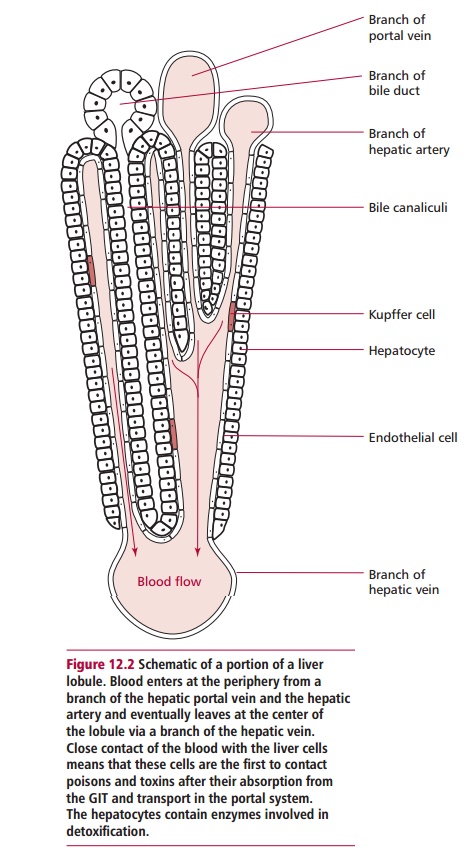
reactions in the liver are favored by its microstructure, which consists of
lobules with a central vein and peripheral branches of the hepatic artery and
the hepatic portal vein. Blood leaves branches of the hepatic artery and
hepatic portal vein and percolates from the periphery of the lobule through
sinusoids, bathing the hepatocytes of the lobule as it flows (Figure 12.2). While it is unusual for
blood to come into such close contact with tissue cells, this arrangement
allows poisons to be removed as the blood flows through the lobule to be
collected by branches of the hepatic vein. Also, mitotic divisions within the
organ replace those liver cells that are irreparably damaged. It goes without
saying that a disease of the liver can compromise its ability to deal with
toxic substances, which can lead to significant clinical consequences.
Detoxification is achieved in two phases. In Phase I, the
xenobiotic is oxidized and/or hydroxylated by mixed function oxidase (MFO)
activities of monooxygenase systems. There are two such systems, a
flavin-containing monooxygenase (FMO) system used in oxidation reactions of
drug metabolism and a cytochrome P-450 monooxygenase system that oxidizes
carbon atoms. Both systems require NADPH as a coenzyme and dioxygen and are
localized in the smooth endoplasmic reticulum. In Phase II, the oxidized
xenobiotic product is linked to a polar compound, such as a glucuronate or
sulfate group forming water-soluble conjugates in further enzyme-catalyzed
reactions. The enzymes that catalyze both the Phase I and II reactions have
broad specificities and are therefore able to detoxify a wide range of organic
toxins and drugs. This is essential since the range of xenobiotics to which the
body may be exposed is enormous, and individual enzyme systems could not be
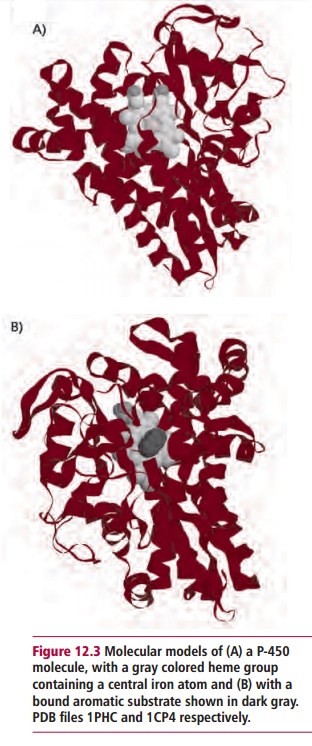
available to deal with each one separately. At least 50 different members of
the cytochrome P-450 enzyme family are found in the smooth endoplasmic
reticulum of hepatocytes (Figure 12.3).
They catalyze the hydroxylation of a wide variety of substances by
incorporating one of the oxygen atoms into the xenobiotic to form the hydroxyl
group,
while the other oxygen atom is reduced to water. The NADPH
supplies an electron to complete the Phase I stage.
R-H + O2 +
NADPH + H+ ® R-OH + H2O + NADP+
The same enzyme system is able to convert unsaturated compounds
to an epoxide, which is a substrate for epoxide hydrolase that catalyzes the
conversion of the epoxide to a glycol.
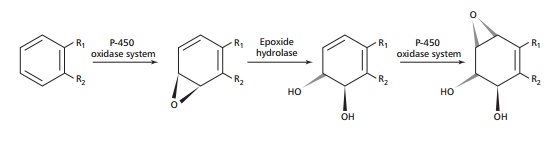
The actions of these enzymes form hydroxyl groups that increase
the water solubility of the original poison or drug and also form attachment points
for the actions of Phase II enzymes. These include glucuronyl transferases of
the smooth endoplasmic reticulum membrane and sulfotransferase in the cytosol,
which use UDP-glucuronate and 3a-phosphoadenosyl 5a-phosphosulfate (PAPS) as the respective donor substrates:
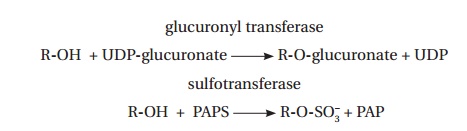
The increased water solubility of the products facilitates their
excretion by the kidneys, although small amounts are also lost in bile to the
feces.
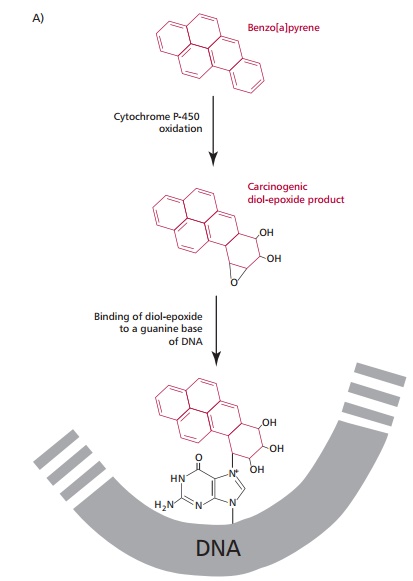
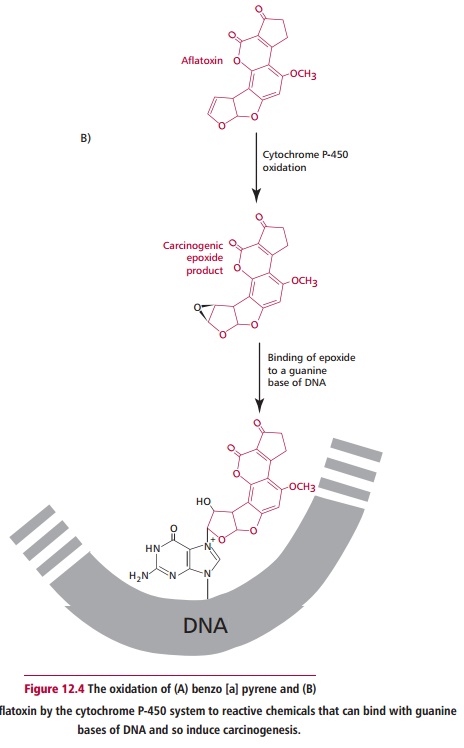
It is unfortunately the case that a number of poisons are
rendered more toxic by oxidation by the P-450 system. Indeed, a number of
fat-soluble compounds that are relatively harmless because they would normally
accumulate in adipose tissue are converted into potentially lethal materials
because of the increase in water solubility. Thus, a number of indirectly
acting carcinogens, for example, benzo[a]pyrene, found in cigarette smoke and the
toxin aflatoxin B1 from Aspergillus
flavus, are converted to highly carcinogenic products (Figure 12.4 (A) and (B)).
Related Topics