Chapter: Biology of Disease: Toxicology
Barbiturates - Toxicology Poisons
BARBITURATES
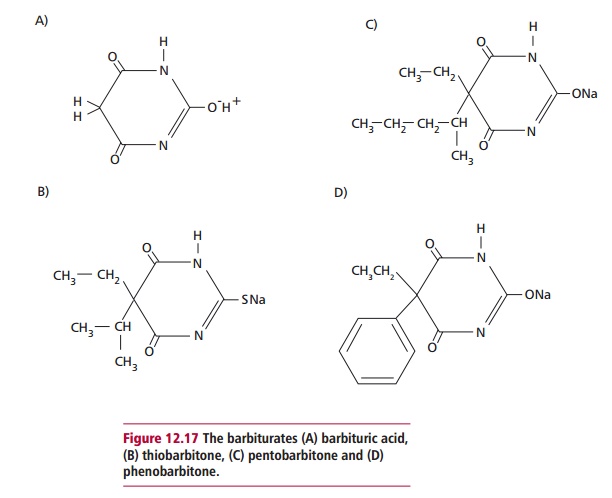
Barbiturates are a group of drugs based on the parent compound,
barbituric acid (Figure 12.17 (A)).
All are sedatives, that is, they depress certain activities of the CNS.
Although they have well-established therapeutic uses, barbiturates can be toxic
if taken as an overdose, indeed, they are one of the commonest methods for
attempting suicide. They were once used as recreational drugs, for example in
purple hearts, and this too led to accidental overdoses. Barbiturates have now
largely been replaced by benzodiazepines and barbiturate overdose is less
likely to be encountered today.
Barbiturates induce drug dependence and this involves three
distinct and independent components: tolerance, physical dependence and
compulsive abuse or psychic craving. Barbiturate dependence stimulates all
three components to such an extent that they produce major problems for the
individual user as well as for society at large. Once barbiturate dependence
has developed, abrupt withdrawal from the drug induces a particularly
unpleasant withdrawal syndrome. This is characterized by weakness, tremors,
anxiety, increased respiratory and pulse rates with a corresponding increased
blood pressure, vomiting, insomnia, loss of weight, convulsions of the grand
mal type and a psychosis resembling alcoholic delirium tremens. Thus during
withdrawal, it is appropriate to reduce the dosage gradually over an extended
period.
The length of time barbiturates act in vivo varies. Some, for example thiobarbitone (Figure 12.17 (B)), are short acting,
others, such as pentobarbitone (Figure
12.17 (C)), act in the medium term, while the group including the best
known barbiturate, phenobarbitone (Figure
12.17 (D)), induce long-lasting effects. Barbiturates act by enhancing the
effects of GABA by binding to a different site on the receptors on target
neurons of the CNS to those for GABA itself. The blood–brain barrier (Figure 3.4) prevents the easy entry of
many substances into the brain compared with their uptake into other tissues.
However, lipid-soluble substances, such as the barbiturate thiopentone, enter
the brain quickly by passive diffusion. This rapid uptake allows thiopentone to
exert its anesthetic effects extremely quickly. In contrast, other
barbiturates, such as phenobarbitone, are weak acids and so may be ionized.
This slows their entry into the CNS but produces a longer lasting effect. The
life-threatening effects of barbiturate poisoning include depression of the
centers in the CNS that control respiration and blood
circulation. Some barbiturates, for example phenobarbitone, can
also induce the synthesis of monooxygenase enzymes and so, by altering the rate
or route of metabolism of other drugs, can alter their toxicities.
There is no antidote for barbiturate poisoning. Primary care is
to maintain a free airway, administer artificial respiration if required and
forced alkaline diuresis. The pH dependence of ionization of many common
barbiturates is exploited by infusions of large volumes of sodium hydrogen
carbonate containing the osmotic diuretics urea or mannitol. This increases the
pH of plasma relative to the cytoplasm of cells and increases the proportion of
ionized barbiturate in the plasma causing more of the un-ionized drug to
diffuse out of the tissues, including the brain, into the plasma. This can
promote a diuresis as large as 12 dm3 in 24 h. The ionized form is
also excreted more rapidly since the alkalinity of the provisional urine
ensures it remains ionized and cannot cross the tubule wall back into the
plasma.
Related Topics