Chapter: Genetics and Molecular Biology: Genetics
Mechanism of a trans Dominant Negative Mutation
Mechanism of a trans Dominant Negative Mutation
Expression
of the lac operon is regulated by the
product of the lacI gene. Since this
protein acts to turn off or turn down the expression, it is called a repressor.
The protein can bind to a site partially overlapping the lac promoter and block transcription from the promoter into the lac struc-tural genes. In the presence
of inducers, the repressor’s affinity for the operator is much reduced and it
dissociates from the DNA. This then permits active transcription of the lac genes.
The
repressor contains four identical subunits. Consider cells that are diploid for
lacI where one of the lacI genes is lacI+, and the other is a type we call lacI-d. The d stands for “dominant.” During the
synthesis of the two types of repressor subunit in a cell, the probability that
four newly synthesized wild-type repressor subunits will associate to form a
wild-type tetramer is low. Instead, most repressor tetramers will possess both
types of subunits.
If the
inclusion of a single I-d subunit in a tetramer interferes with the
function of a tetramer, then the I-d allele will be dominant and act
in trans to nullify the activity of
the good lacI allele, that is, it is
a trans dominant negative mutation.
What is
the physical basis for a single defective subunit inactivating the remaining
three nondefective subunits in a tetramer? Lac repressor contacts the
symmetrical lac operator with two
subunits utilizing the helix-turn-helix structure that was discussed. Contact
with only one subunit provides far too little binding energy for the protein to
bind. Therefore a subunit with a defective DNA-contacting domain may be capable
of folding and oligomerizing with normal subunits, but its incorporation into a
tetramer will interfere with DNA binding since two good subunits must
simultaneously be involved in contacting the opera-tor.
If a
tetramer contains only one defective subunit, we might think that two
nondefective subunits could still be utilized for binding to DNA. To an extent
this is true. However, just as enhancers loop and complexes of proteins can
contact the DNA at two or more sites, so does the lac
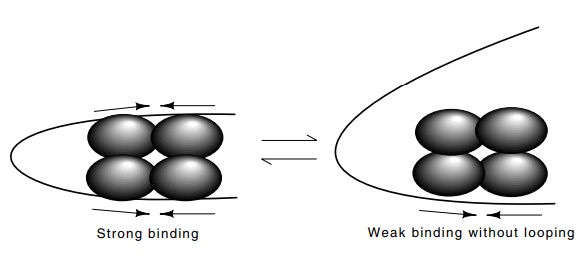
Figure
8.6 When all four subunits oflacrepressor can bind DNA and form
theloop, binding to both of the DNA sites is enhanced because looping
effectively increases the local concentration of the repressor at the other
sites.
operon. Looping in the lac operon brings repressor bound at the primary operator into
contact with either of two so-called pseudo-operators on either side. As will
be discussed later, such looping can greatly increase the occupancy of a
binding site by a protein. In the case of the tetrameric lac repressor, two good subunits could contact the lac operator or apseudo operator, but
looping could not occur, and the overall binding would be relatively weak and
as a result, repression would be poor (Fig. 8.6). Thus, the inclusion of a
single DNA-binding defective subunit in a tetrameric repressor can greatly
interfere with repression.
Related Topics