Chapter: Basic & Clinical Pharmacology : Pharmacologic Management of Parkinsonism & Other Movement Disorders
Levodopa
LEVODOPA
Dopamine does not
cross the blood-brain barrier and if given into the peripheral circulation has
no therapeutic effect in parkinsonism. However, (–)-3-(3,4-dihydroxyphenyl)-L-alanine (levodopa),
the immediate metabolic precursor of dopamine, does enter the brain
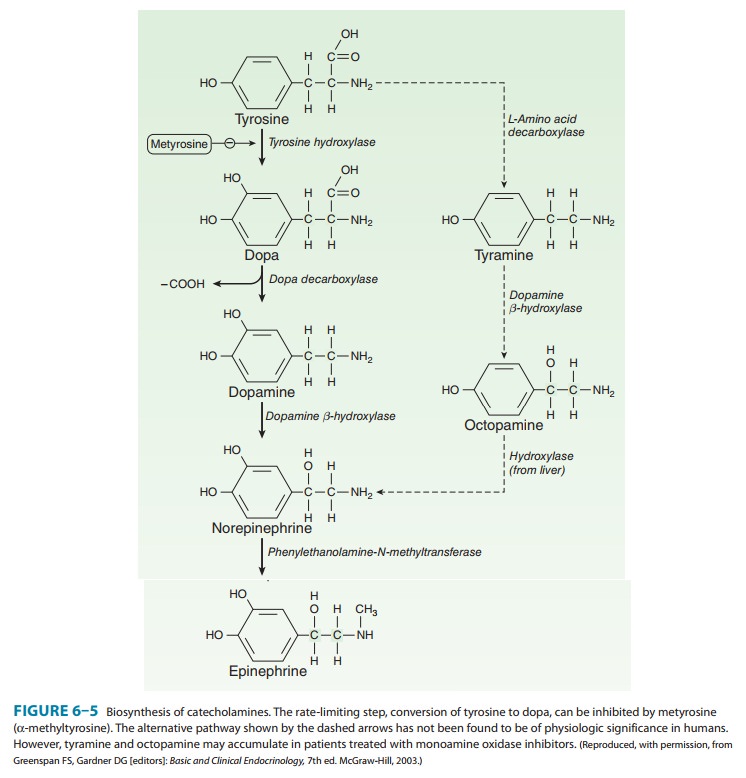
Several noncatecholamine dopamine receptor agonists have also been developed and may lead to clinical benefit, as discussed in the text that follows. Dopamine receptors of the D1 type are located in the pars compacta of the substantia nigra and presynaptically on striatal axons coming from cortical neurons and from dopaminergic cells in the substantia nigra. The D2 receptors are located postsynapti-cally on striatal neurons and presynaptically on axons in the sub-stantia nigra belonging to neurons in the basal ganglia. The benefits of dopaminergic antiparkinsonism drugs appear to depend mostly on stimulation of the D2 receptors. However, D1- receptor stimulation may also be required for maximal benefit and one of the newer drugs is D3 selective. Dopamine agonist or partial agonist ergot derivatives such as lergotrile and bromocrip-tine that are powerful stimulators of the D2 receptors have antipar-kinsonism properties, whereas certain dopamine blockers that are selective D2 antagonists can induce parkinsonism.
Chemistry
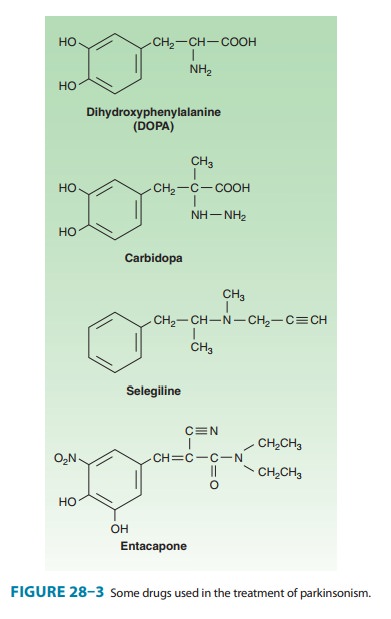
Dopa is the amino acid
precursor of dopamine and norepineph-rine. Its structure is shown in Figure
28–3. Levodopa is the levorotatory stereoisomer of dopa.
Pharmacokinetics
Levodopa is rapidly
absorbed from the small intestine, but its absorption depends on the rate of
gastric emptying and the pH of the gastric contents. Ingestion of food delays
the appearance of levodopa in the plasma. Moreover, certain amino acids from
ingested food can compete with the drug for absorption from the gut and for
transport from the blood to the brain. Plasma concen-trations usually peak
between 1 and 2 hours after an oral dose, and the plasma half-life is usually
between 1 and 3 hours, although it varies considerably among individuals. About
two thirds of the dose appears in the urine as metabolites within 8 hours of an
oral dose, the main metabolic products being 3-methoxy-4-hydroxyphenyl acetic
acid (homovanillic acid, HVA) and dihy-droxyphenylacetic acid (DOPAC).
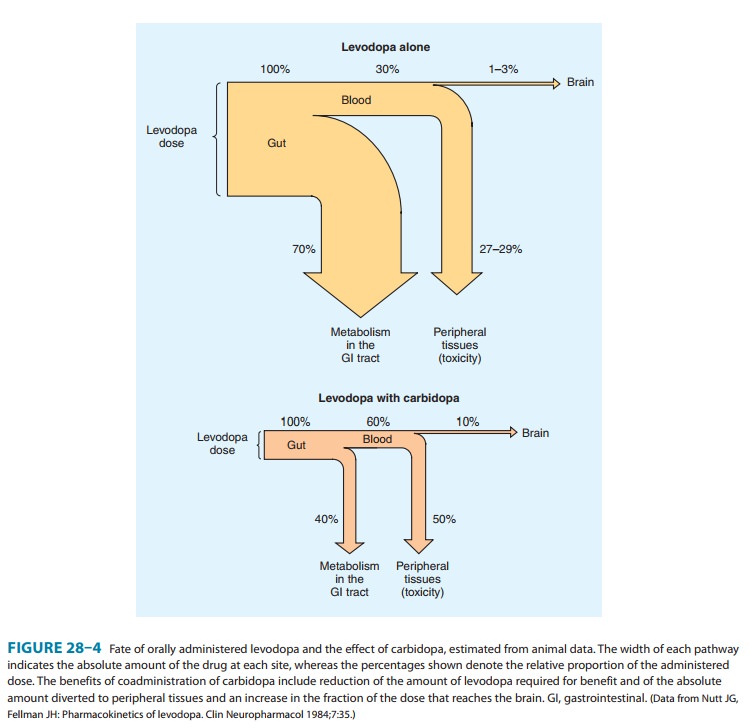
Unfortunately, only about 1–3% of administered levodopa actually enters the
brain unal-tered; the remainder is metabolized extracerebrally, predominantly
by decarboxylation to dopamine, which does not penetrate the blood-brain
barrier. Accordingly, levodopa must be given in large amounts when used alone.
However, when given in combination with a dopa decarboxylase inhibitor that
does not penetrate the blood-brain barrier, the peripheral metabolism of
levodopa is reduced, plasma levels of levodopa are higher, plasma half-life is
longer, and more dopa is available for entry into the brain (Figure 28–4).
Indeed, concomitant administration of a periph-eral dopa decarboxylase
inhibitor such as carbidopa may reduce the daily requirements of levodopa by
approximately 75%.
Clinical Use
The best results of
levodopa treatment are obtained in the first few years of treatment. This is
sometimes because the daily dose of levodopa must be reduced over time to avoid
adverse effects at doses that were well tolerated initially. Some patients
become less responsive to levodopa, perhaps because of loss of dopaminergic
nigrostriatal nerve terminals or some pathologic process directly involving
striatal dopamine receptors. For such reasons, the benefits of levodopa
treat-ment often begin to diminish after about 3 or 4 years of therapy,
regardless of the initial therapeutic response. Although levodopa therapy does
not stop the progression of parkinsonism, its early ini-tiation lowers the
mortality rate. However, long-term therapy may lead to a number of problems in
management such as the on-offphenomenon discussed below. The most appropriate
time to intro-duce levodopa therapy must therefore be determined individually.
When levodopa is used,
it is generally given in combination with carbidopa (Figure 28–3), a peripheral
dopa decarboxylase inhibitor, which reduces peripheral conversion to dopamine.
Combination treatment is started with a small dose, eg, carbi-dopa 25 mg,
levodopa 100 mg three times daily, and gradually increased. It should be taken
30–60 minutes before meals. Most patients ultimately require carbidopa 25 mg,
levodopa 250 mg three or four times daily. It is generally preferable to keep
treat-ment with this agent at a low level (eg, carbidopa-levodopa 25/100 three
times daily) when possible, and to use a dopamine agonist instead, to reduce
the risk of development of responsefluctuations. A controlled-release formulation of
carbidopa-levodopa is available and may be helpful in patients with
estab-lished response fluctuations or as a means of reducing dosing frequency.
A formulation of carbidopa-levodopa (10/100, 25/100, 25/250) that disintegrates
in the mouth and is swal-lowed with the saliva (Parcopa) is now available commercially and is best taken about 1
hour before meals. The combination (Stalevo)
of levodopa, carbidopa, and a catechol-O-methyltrans-ferase
(COMT) inhibitor (entacapone) is discussed in a later section. Finally, therapy
by intraduodenal infusion of
levodopa-carbidopa appears to be safe and is superior to a number of oral
combination therapies in patients with response fluctuations. This approach has
been used to a greater extent in Europe than the USA, but interest is growing.
Levodopa can
ameliorate all the clinical features of parkin-sonism, but it is particularly
effective in relieving bradykinesia and any disabilities resulting from it.
When it is first introduced, about one third of patients respond very well and
one third less well. Most of the remainder either are unable to tolerate the
medication or simply do not respond at all, especially if they do not have
clas-sic Parkinson’s disease.
Adverse Effects
A. Gastrointestinal Effects
When levodopa is given without a peripheral decarboxylase inhibitor, anorexia and nausea and vomiting occur in about 80% of patients. These adverse effects can be minimized by taking the drug in divided doses, with or immediately after meals, and by increasing the total daily dose very slowly. Antacids taken 30–60 minutes before levodopa may also be beneficial. The vomiting has been attributed to stimulation of the chemoreceptor trigger zone located in the brainstem but outside the blood-brain barrier. Fortunately, tolerance to this emetic effect develops in many patients. Antiemetics such as phenothiazines should be avoided because they reduce the antiparkinsonism effects of levodopa and may exacerbate the disease.
When levodopa is given
in combination with carbidopa, adverse gastrointestinal effects are much less
frequent and trouble-some, occurring in less than 20% of cases, so that
patients can tolerate proportionately higher doses.
B. Cardiovascular Effects
A variety of cardiac
arrhythmias have been described in patients receiving levodopa, including
tachycardia, ventricular extrasysto-les and, rarely, atrial fibrillation. This
effect has been attributed to increased catecholamine formation peripherally.
The inci-dence of such arrhythmias is low, even in the presence of estab-lished
cardiac disease, and may be reduced still further if the levodopa is taken in
combination with a peripheral decarboxylase inhibitor.Postural hypotension is
common, but often asymptomatic, and tends to diminish with continuing
treatment. Hypertension may also occur, especially in the presence of
nonselective monoamine oxidase inhibitors or sympathomimetics or when massive
doses of levodopa are being taken.
C. Behavioral Effects
A wide variety of
adverse mental effects have been reported, including depression, anxiety,
agitation, insomnia, somnolence, confusion, delusions, hallucinations,
nightmares, euphoria, and other changes in mood or personality. Such adverse
effects are more common in patients taking levodopa in combination with a
decarboxylase inhibitor rather than levodopa alone, presumably because higher
levels are reached in the brain. They may be pre-cipitated by intercurrent
illness or operation. It may be necessary to reduce or withdraw the medication.
Several atypical antipsy-chotic agents that have low affinity for dopamine D2 receptors (clozapine,
olanzapine, quetiapine, and risperidone;) are now available and may be
particularly helpful in counteracting such behavioral complications.
D. Dyskinesias and Response Fluctuations
Dyskinesias occur in
up to 80% of patients receiving levodopa therapy for more than 10 years. The
character of dopa dyskinesias varies between patients but tends to remain
constant in individual patients. Choreoathetosis of the face and distal
extremities is the most common presentation. The development of dyskinesias is
dose related, but there is considerable individual variation in the dose
required to produce them.Certain fluctuations in clinical response to levodopa
occur with increasing frequency as treatment continues. In some patients, these
fluctuations relate to the timing of levodopa intake (wearing-off reactions or end-of-dose
akinesia). In other instances, fluctuations in clinical state are unrelated
to the timing of doses (on-off phenomenon).
In the on-off phe-nomenon, off-periods of marked akinesia alternate over the
course of a few hours with on-periods of improved mobility but often marked
dyskinesia. For patients with severe off-periods who are unresponsive to other
measures, subcutaneously injected apomorphine may provide temporary benefit.
The phenomenon is most likely to occur in patients who responded well to
treatment initially. The exact mechanism is unknown. The dyskinesias may relate
to an unequal distribution of stri-atal dopamine. Dopaminergic denervation plus
chronic pulsa-tile stimulation of dopamine receptors with levodopa has been
associated with development of dyskinesias. A lower incidence of dyskinesias
occurs when levodopa is administered continu-ously (eg, intraduodenally or
intrajejunally), and with drug delivery systems that enable a more continuous
delivery of dopaminergic medication.
E. Miscellaneous Adverse Effects
Mydriasis may occur
and may precipitate an attack of acute glaucoma in some patients. Other reported
but rare adverse effects include various blood dyscrasias; a positive Coombs’
test with evidence of hemolysis; hot flushes; aggravation or pre-cipitation of
gout; abnormalities of smell or taste; brownish discoloration of saliva, urine,
or vaginal secretions; priapism; and mild—usually transient—elevations of blood
urea nitro-gen and of serum transaminases, alkaline phosphatase, and bilirubin.
Drug Holidays
A drug holiday
(discontinuance of the drug for 3–21 days) may temporarily improve responsiveness
to levodopa and alleviate some of its adverse effects but is usually of little
help in the man-agement of the on-off phenomenon. Furthermore, a drug holiday
carries the risks of aspiration pneumonia, venous thrombosis, pulmonary
embolism, and depression resulting from the immobil-ity accompanying severe
parkinsonism. For these reasons and because of the temporary nature of any
benefit, drug holidays are not recommended.
Drug Interactions
Pharmacologic doses of
pyridoxine (vitamin B6) enhance the extracerebral metabolism of levodopa and may
therefore prevent its therapeutic effect unless a peripheral decarboxylase
inhibitor is also taken. Levodopa should not be given to patients taking
monoamine oxidase A inhibitors or within 2 weeks of their dis-continuance
because such a combination can lead to hypertensive crises.
Contraindications
Levodopa should not be
given to psychotic patients because it may exacerbate the mental disturbance.
It is also contraindicated in patients with angle-closure glaucoma, but those
with chronic open-angle glaucoma may be given levodopa if intraocular pres-sure
is well controlled and can be monitored. It is best given combined with
carbidopa to patients with cardiac disease; even so, the risk of cardiac
dysrhythmia is slight. Patients with active peptic ulcer must also be managed
carefully, since gastrointestinal bleed-ing has occasionally occurred with
levodopa. Because levodopa is a precursor of skin melanin and conceivably may
activate malig-nant melanoma, it should be used with particular care in
patients with a history of melanoma or with suspicious undiagnosed skin
lesions; such patients should be monitored by a dermatologist regularly.
Related Topics