Characteristics, Structure, Enzyme Regulation, Uses of Microbial Enzymes | Microbial Metabolism - Enzymes | 12th Microbiology : Chapter 4 : Microbial Metabolism
Chapter: 12th Microbiology : Chapter 4 : Microbial Metabolism
Enzymes
Enzymes
Life is
an intricate meshwork involving a perfect coordination of a vast majority of
chemical reactions. This is due to the presence of some catalysts synthesized
inside the body of the organism. The term ‘enzyme’ was coined by Friedrich
Wilhen Kuhne (1878) to designate these biological catalysts. The name ‘enzyme’
(en – in, zyme – yeast) literally means ‘in yeast’. The name of enzyme usually
ends in – ase. Example: Cytochrome dehydrogenase. The study of enzyme is called
Enzymology.
Enzymes
are proteins or large biomolecules that can catalyze certain biochemical
reactions for metabolic process within the cell. The substances that can speed
up a chemical reaction without being permanently altered itself are called
catalysts. Enzymes accelerate the rate of chemical reactions. The molecule upon
which enzyme may act are called substrate and the enzyme convert the substrate
into different molecules known as products. The enzyme serves as biological
catalyst (Table 4.3).
Tyrosinases are syn- thesized by Agaricus bisporus, which is involved in melano-genesis
(pigmentation of skin and hair).
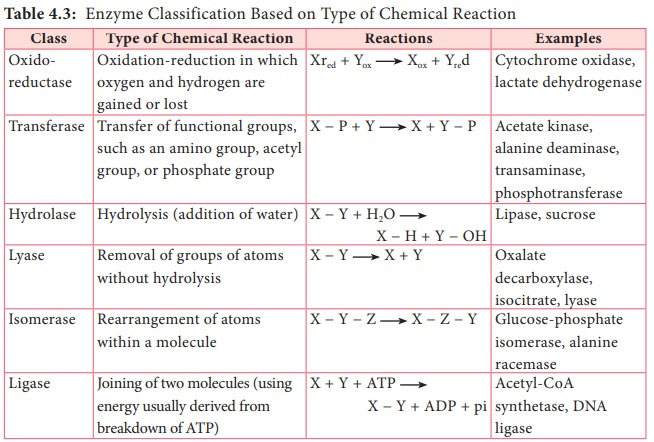
Table 4.3: Enzyme Classification Based on Type of Chemical Reaction
Characteristics of Enzymes
Enzymes
• are highly substrate specific
• are reused at several times
• synthesized within the cells are determined by genes
• speed
up the chemical reaction
• decrease the activation energy needed to start
• act as
a biocatalyst
Infobits
Proteins have four levels of structure (i) primary (sequence of amino acids), (ii) secondary (regular coils or pleats linked by peptide bonds), (iii) tertiary overall three dimensional structure of a polypeptide linked by disulphide bonds) and (iv) quatenary structure (two or more polypeptides chains). Like all proteins, enzymes are composed of one or more long chain of inter connected amino acids.
Low level of catalase plays a major
role in greying process of human hair.
Structure of Enzymes
Enzymes are generally globular proteins that range
in molecular weight from about 10,000 to several million. Each enzyme possesses
a unique sequence of amino acid that causes it to fold into a characteristic
three dimensional shape with a specific surface configuration. This enables it
to find the correct substrate from large number of diverse molecules in the
cell.
A molecule acted upon by an enzyme is called a
substrate. Enzymes are specific and act on specific substrates and each enzyme
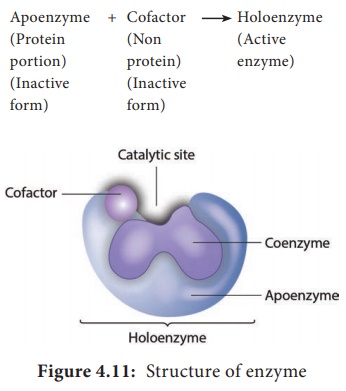
catalyzes only one reaction. Enzyme consists of a protein portion, named
apoenzyme and a non protein component, named cofactor (Figure 4.11).
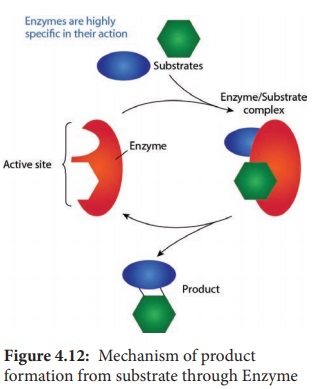
The
region of an enzyme where substrate molecules bind and undergo a chemical
reaction is its active site. Each active site is specially designed in response
to their substrate; as a result most enzymes have specificity and can only
react with particular substances. After the formation of enzyme substrate
complex (Figure 4.12), forces exerted on the substrate by the enzyme cause it
to react and become the product of the intended reaction.
Example: Sucrase catalyses the hydrolysis of
sucrose to glucose and fructose.
Apoenzyme
is the inactive form of the enzyme which gets activated after binding with a
cofactor. Coenzymes are small organic molecules that can be loosely bound to an
apoenzyme and they transport chemical group from one enzyme to another.
Cofactor
is a chemical compound or metallic ion that is required for enzyme activity.
Example: NAD+ is derived from vitamin B. Some cofactors are metal
ions including iron (Fe), copper (Cu), magnesium (Mg), manganese (Mn), Zinc
(Zn), calcium (Ca) and cobalt. If the cofactor is tightly or firmly attached to
the apoenzyme it is called a prosthetic group. The prosthetic group may be
organic [such as vitamin, sugar, and lipid] or inorganic [such as metal ion]
but is not composed of amino acids.
The complete enzyme consisting of the apoenzyme and its cofactor is called the holoenzyme
Microbial Enzymes
Many
microbes synthesize and excrete large quantities of enzymes into the
surrounding medium. Using this feature of these tiny organisms many enzymes
like Amylase, Cellulase, Catalase, Protease, and Lipase are produced
commercially.
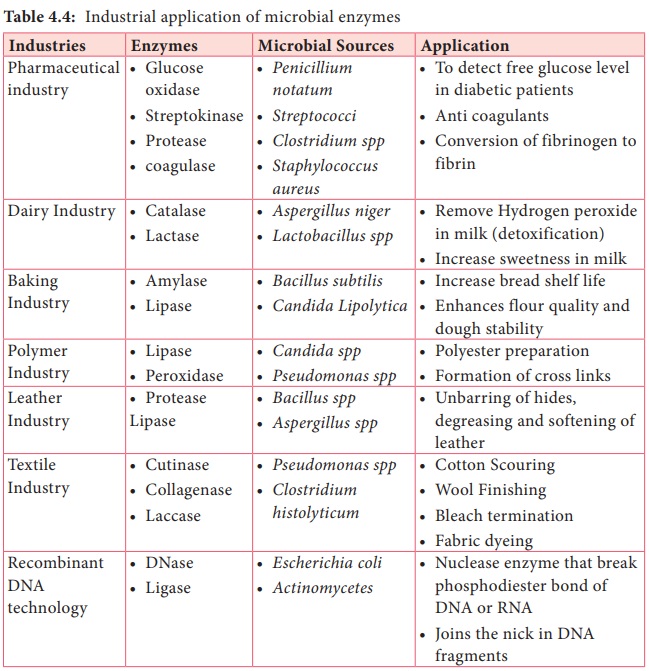
Microbial enzymes are extensively used in food
processing, preservation, washing powder preparation, leather industry, and
paper industry and in scientific research (Table 4.4).
Table
4.4:
Industrial application of microbial enzymes
Infobits
Idoenella sakaiensis is a bacterium capable of breaking down PET plastics. The bacterium first uses
PETase to break down the PET plastic. This has potential importance in the
recycling process of PET plastics.
Lipase is used in the determination of triglyceride and blood cholesterol
level. Lipase producing microorganism have been found in industrial wastes,
vegetable oil processing factories, diary plants and soil contaminated with
oil.
Enzyme Regulation
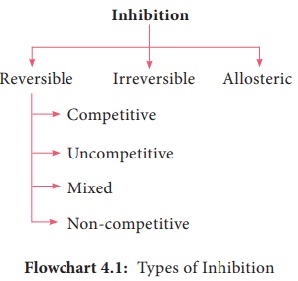
Inhibitors:
An enzyme inhibitor is a molecule that binds to an enzyme and decreases its
activity (Flowchart 4.1). This adverse affect of inhibitors on the rate of
enzymatically catalyzed reactions are called inhibition.
An irreversible
inhibitor inactivates an enzyme by binding covalently to a particular group at
the active site. A reversible inhibitor inactivates an enzyme by non covalent,
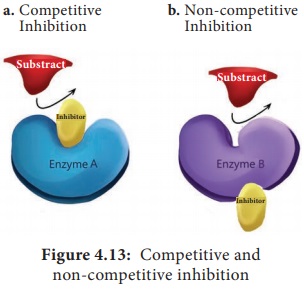
more easily reversible interactions. Competitive inhibitor is any compound that
bears a structural resemblance to a particular substrate for binding at the
active site of an enzyme. Non competitive inhibitors do not compete with the
substrate for the enzyme’s active site; instead, they interact with another
part of the enzyme. Uncompetitive inhibitors bind only to the enzyme substrate
complex without binding to the free enzyme (Figure 4.13).
Administration of the enzyme DNase I to the lungs of cystic fibrosis
patients decrease the viscosity of the mucus and the breathing is made easier.
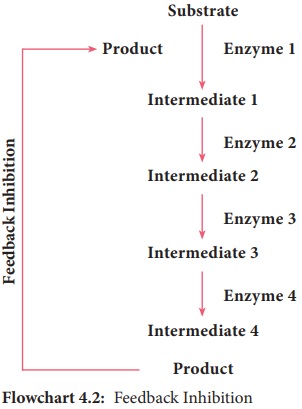
Feedback inhibition
In
Feedback inhibition, the final product allosterically inhibits the enzyme that
catalyses the first stage in the series of reactions. This process is used to
regulate the synthesis of amino acids (Flowchart 4.2). Example: Threonine
deaminase is the first enzyme in the conversion of Threonine to Isoleucine.
Isoleucine inhibits Threonine deaminase through feedback inhibition.
Uses of Microbial Enzymes
Microbial
enzymes are
• helpful to save energy and prevent pollution
• highly
specific
• be
immobilized and reused
• inexpensive
and more stable
• easily
extracted and purified
• genetically
manipulated to yield higher quality
Related Topics