Microbial Metabolism | Microbiology - Energy of Chemical Reaction | 12th Microbiology : Chapter 4 : Microbial Metabolism
Chapter: 12th Microbiology : Chapter 4 : Microbial Metabolism
Energy of Chemical Reaction
Energy of Chemical Reaction
Light
energy is trapped by phototrophs during photosynthesis, in which it is absorbed
by bacteriochlorophyll and other pigments and converted to chemical energy for
cellular work. The energy is required by the bacterium for synthesis of cell
wall or membrane, synthesis of enzymes, cellular components, repair mechanism,
growth and reproduction.
Some change of energy occurs whenever bonds between
atoms are formed or broken during chemical reactions. When a chemical bond is
formed, energy is required. Such a chemical reaction which requires energy is
called an endergonic reaction (energy is directed inward). When a bond is
broken, energy is released. A chemical reaction that release energy is an
exergonic reaction (energy is directed outward).
During chemical reaction energy is either released
or absorbed and the quantum of energy liberated or taken up is useful energy
and is referred to Free Energy Change (ΔG) of the reactions.
High Energy Phosphate
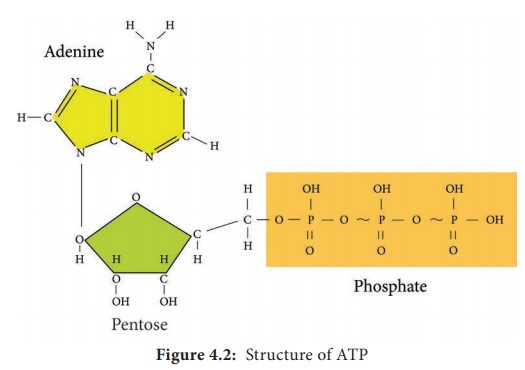
Adenosine
Tri-Phosphate (ATP) is the principal energy carrying molecule of all cells and
is indispensable to the life of the cell. It stores the energy released by some
chemical reactions, and it provides the energy for reactions that require
energy. ATP consists of an adenosine unit composed of adenine, ribose with
three phosphate groups. In ATP and some other phosphorylated compounds, the
outer two phosphate groups are joined by an anhydride bond.
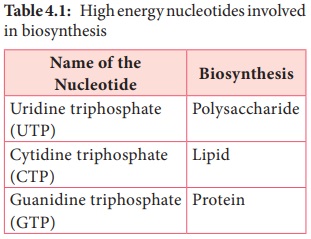
Some of
the other high energy nucleotides involved in biochemical processes are given
in Table 4.1.
Table 4.1: High energy nucleotides involved in biosynthesis
Nutrients
are broken from highly reduced compounds to highly oxidized compounds within
the cells. Much of the energy released during oxidation - reduction reactions
is trapped within the cell by the formation of ATP. A phosphate group is added
to ADP with the input of energy to form ATP.
ATP + H2O
→ ADP + pi
(ΔG° = −7.3 K Cal/mol)
ATP + H2O
→ AMP +
ppi (ΔG° = −10.9 K Cal/mol)
ATP is
ideally suited for its role as an energy currency. It is formed in energy
trapping and energy generating processes such as photosynthesis, fermentation,
and aerobic respiration. In bacterial and archeal cells, most of the ATP is
formed on the cell membrane, while in eukaryotes the reactions occur primarily
in the mitochondria (Figure 4.2)
Oxidation – Reduction Reactions
Oxidation
is the removal of electrons (e−) from an atom or molecule and is often an
energy producing reaction. Reduction of a substrate refers to its gain or
addition of one or more electrons to an atom or molecule. Oxidations and
reduction are always coupled. In other words, each time one substance is
oxidized, another is simultaneously reduced.
F2
+ 2e− → 2F−
H2 + 2e− → 2H+ + 2e−
NAD+
+ 2H+ + 2e− ↔ NADH + H+
Related Topics