Chapter: Medicine Study Notes : Reproductive and Obstetrics
Breast Cancer
Breast Cancer
Epidemiology
·
In NZ, 1600 cases each year, 580
die. Commonest cause of cancer death in
women.
·
10% life time incidence (usually
over 70)
·
Maori rate similar to non-Maori
·
75% diagnosed with breast cancer
are over 50. Uncommon under 40. Mean age of diagnosis is 60 – 65. Younger if
genetic risk
·
If > 70 years, more likely to
be indolent and hormone responsive. If
< 35 then large and aggressive
·
Survival:
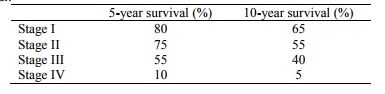
·
Risk Factors
·
Major risks:
o Woman (100 * men)
o Age
o Previous breast cancer, also previous (or family) history of
endometrial, prostate, or ovarian cancer
o Biopsy showing an at risk condition e.g. atypical hyperplasia
o Genetic predisposition (eg BRAC1 or 2 account for 5% of breast cancers)
o Family History:
§ Most with family history don‟t develop it, most who get it won‟t have a
family history
§ Risk is above population risk for only 1% of female population
§ 4% have a moderate increase in risk if:
· A mother, sister or father developed breast cancer before 50, or in both breasts
·
More than one close relative on
the same side of the family who had breast or ovarian cancer (geneticist said
only genetic if 3 or more affected relatives – it is so common have to have a
high incidence in family before suspecting a family loading)
·
Minor risks:
o Oestrogen exposure:
§ Slight increase for OC and Depo-Provera (only while taking it – and
usually young so less of an issue)
§ Longer duration between menarche and menopause
§ First child beyond 35 or no children
o Not having lactated ® slight risk of premenopausal cancer
o Obesity
o HRT for more than 5 years increases risk by about 30%. Risk disappears
within 5 years of stopping
o Radiation, environmental hazards
·
Not risk factors:
o Smoking
o Small (now disproven?) relationship with low fat, high fibre diet
Symptoms
·
Presenting symptoms:
o Painless mass: 66%
o Painful mass: 11%
o Nipple discharge: 9%
·
Usual presentation is a dominant,
painless mass
·
New lump or thickening
·
Change in breast shape or size
·
Puckering or dimpling of the skin
·
Change in a nipple
·
Lumpiness in one breast soon
after period ends
·
Pain in the breast that is
unusual
Investigations
·
History and clinical exam
·
Mammogram:
o Not sensitive < age 35
o Calcifications: low risk are coarse or rounded, high risk are clustered
or branching
o Shadows: malignant are less circumscribed
·
Ultrasound
·
FNA ® Cytology
·
Core or hook wire biopsy
Pathogenesis
· Most tumours occur in the epithelial component lining the ducts and lobules. Epithelial hyperplasia (1 – 2 times risk) ® Atypical hyperplasia – proliferation and atypia of ductal or lobular epithelium. Risk of subsequent cancer = 4 times.
·
Tumour cells secrete cytokines ® fibrosis
® lump. Easier to detect in an older woman (fat and ¯intra-lobular
fibrosis)
·
All breast cancers are different.
Tumour growth rates vary considerably. On average takes 9 years to reach 1 cm.
·
Death is from metastases which
can occur at any time
·
Spreads to lymph nodes via
lymphatics and directly to distant
sites via blood stream – not via lymph nodes then to distant sites (although lymph
node involved Þ risk of blood spread as well)
·
Lots of implicated genes. Those in familial breast cancer include:
o BRAC1:
·
Autosomal dominant (but recessive
at the level of the cell): if carrier then 65 – 75% risk (ie high penetrance)
·
A tumour suppressor gene,
expressed in breast, ovary, thymus, testis
·
Accounts for 40 – 50% of familial
breast cancer
o BRAC2:
·
Associated with male breast
cancer, not ovarian
·
10% of inherited breast cancer
Classification of Breast Cancer
·
Classification:
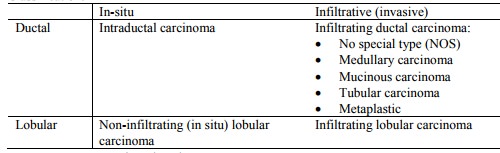
o Most cancers are intraductal
o Plus Paget‟s Disease of the Nipple
·
Non-infiltrating/in-situ breast cancer: Does
not metastasise but recurrence is a problem. Can become infiltrative and then metastasise
o Intraductal carcinoma (20 – 30%):
§ Comedocarcinoma: solid intraductal proliferation, central necrosis, microcalcifications on mammogram
§ Classified by nuclear grade (low, intermediate and high) and the presence or absence of necrosis.
§ Can eventually become invasive: removal ® cure
o Paget‟s disease (a type of ductal carcinoma in situ): lesion of the nipple caused by malignant cells arising from ducts and invading the nipple epithelium. Looks inflamed (early on can look like eczema). Most often an underlying duct carcinoma.
o Lobular carcinoma in situ:
§ Usually an incidental finding on biopsy affecting terminal ductules
§ Proliferation of terminal ductules and acini
§ 1% per year risk of invasive carcinoma in same or opposite breast – removal isn‟t necessarily cure
·
Invasive/infiltrating breast cancer:
o Main risk factor: age
o Infiltrative ductal carcinoma (65 – 80%):
§ No special type: Most common. Grossly stellate or multinodular and very
hard. Histologically compressed ductules in a very desmoplastic stroma
§ Medullary: Big, bulky and soft, plentiful lymphocytes, better prognosis than other types
§ Mucinous (colloid, gelatinous) carcinoma: Grossly: gelatinous mass.
Histologically: clumps of cells in lakes of mucin. Better prognosis
§ Tubular Carcinoma: well-formed glands, best prognosis
o Infiltrative lobular carcinoma:
§ Histological: Indian files around ducts, small cells
§ Often bilateral
·
Features of invasive cancers:
o Usually dominant mass
o Usually painless
o In time fixed to deep fascia ® immobile
o Orange peel appearance: blocked lymphatics ® oedema +
suspensory ligaments contract ® distorted shape
o Also nipple retraction, ulceration of overlying skin
o Majority arise in the outer quadrants – particularly the upper, outer
quadrant
·
On mammography:
o Infiltrative edge: not well demarcated
o Density
compared with adipose tissue
o Micro-calcifications: small clustered areas of necrosis
Prognosis
·
Stage: axillary metastases most
important, also size. Cancers found on mammography or by self-examination are
smaller Þ better prognosis
·
Grade
·
Oestrogen receptor sensitivity:
if positive then better – more differentiated and Tamoxifen ®
regression
Treatment of Breast Cancer
·
Can‟t cure metastases ® aim of
treatment is local control
·
Options:
o Two options (similar long-term survival):
§ Removal of the lump + radiation therapy (significant ¯ in local
recurrence)
§ Mastectomy (or radical mastectomy) + reconstruction
o +/- Radiotherapy (planned to limit dose to the heart, lung or opposite
breast
o +/- Tamoxifen (anti-oestrogen)
·
Surgery:
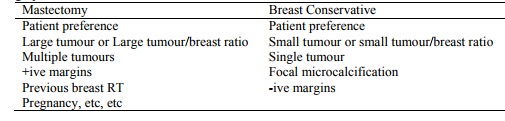
·
Most common metastasis is in the
bone. Bisphosphonates ® slow
osteolysis
·
Risk factors for recurrence in
breast cancer (Þ consider adjuvant chemo):
o Axillary node status (strongest predictor)
o Tumour size (> 1 cm)
o Histological tumour type and grade
·
Adjuvant Chemotherapy:
o Approx 25 – 30% ¯ risk of recurrence, 15 – 20% ¯ risk of death. Improves long term survival in node positive and node negative disease
o 4 to 6 courses over 3 – 6 months optimal
o 2 agents better than one: eg
§ AC: Adriamycin (an anthracycline) and Cyclophosphamide. „Gold standard‟.
Adriamycin causes vomiting and wasn‟t used so much until 5HT3 antagonists were
available
§ CMF: Cyclophosphamide, Methotrexate and Fluorouracil (another „Gold
Standard‟)
·
Hormone Therapy:
o Aim: prevent breast cancer cells from receiving stimulation from
oestrogen
o Only is oestrogen receptor sensitive
o Oestrogen deprivation:
§ Block oestrogen receptor: eg Tamoxifen – antagonist. Taken for 5 years. Side-effects:
·
Largely well tolerated
·
1 in 3 have post menopausal
flushes, vaginal dryness/discharge
·
Initial nausea, weight gain
· Rare retinopathy
· Agonist in the uterus ® endometrium ® risk of endometrial carcinoma (1 in 1000, usually curable)
· PE/DVT (1 – 2 %)
§ Suppress synthesis: aromatase inhibitors (work in adipose tissue, eg in
post menopausal women), LHRH agonist (pre-menopausal, switches off the ovary)
§ Destroy ovaries (surgery or RT)
o Leads to ¯recurrent, ¯ 40% incidence of contralateral breast cancer (although absolute risk
low)
Breast Screening
·
Of proven benefit in reducing
mortality in women over 50: benefits under 50 unclear
·
2 yearly screening after 50 reduces
chances of dying from breast cancer by about 1/3. Reduces a one in 42 chance to
one in 60
·
In NZ is free from 50 – 64
·
Mammograms less reliable in under
50s: denser breast tissue. Higher false positives ® unnecessary
investigations. Sensitivity for < 50 years is 50% - 60%, for > 50 years
is 80+%. 5 – 10 % screened sent for further investigations. Positive
predicative yield is 8.5% (high false positive rate)
·
Further investigations:
ultrasound, FNA, biopsy
·
Of 1000 screened, 70 to 120 will
be positive, 10 to 30 will proceed as far as open biopsy, and 5 to 10 will have
cancer
·
Mammogram less accurate if on HRT
·
Interval cancers: fast growing
cancers appearing between mammograms – never ignore a lump
·
Application of screening criteria
(see Criteria for Screening Programmes, page 690):
o It is an important health problem – with a significant incidence. It is preventable
o A screening test is available: a two yearly double view double read
mammography (double reading increases cancer detection by 15% compared with
single reading and reduces recall rate)
o The screening test is available, acceptable (83% a little uncomfortable
only), reasonable sensitivity, but low PPV
o Natural history is well understood, and there is a detectable
pre-symptomatic stage
o Screening leads to interventions that increase the quality of life: relative risk reduction 10 – 30% for women in the 50 – 65 age group. However, lots of unnecessary interventions, and for a majority (>70%) whose cancer is diagnosed, the outcome is unchanged (but will live with 2 years extra knowledge of condition)
o Is there an appropriate infrastructure to provide screening and
follow-up? There have been pilot studies
o Is it cost effective: Needs at
least 70% screening coverage to be cost effective.
Related Topics