Chapter: Medical Surgical Nursing: Oncology: Nursing Management in Cancer Care
Biologic Response Modifiers - Management of Cancer
BIOLOGIC
RESPONSE MODIFIERS
Biologic response
modifier (BRM) therapy involves the use
ofnaturally occurring or recombinant (reproduced through genetic engineering)
agents or treatment methods that can alter the im-munologic relationship
between the tumor and the cancer patient (host) to provide a therapeutic
benefit. Although the mechanisms of action vary with each type of BRM, the goal
is to destroy or stop the malignant growth. The basis of BRM treatment lies in
the restoration, modification, stimulation, or augmentation of the body’s
natural immune defenses against cancer.
Nonspecific Biologic Response Modifiers
Some of the early investigations of the stimulation of the immune system
involved nonspecific agents such as Bacille Calmette-Guérin (BCG) and Corynebacterium parvum. When injected
into the patient, these agents serve as antigens that stimulate an im-mune
response. The hope is that the stimulated immune system will then eradicate
malignant cells. Extensive animal and human investigations with BCG have shown
promising results, especially in treating localized malignant melanoma.
Additionally, BCG is considered to be a standard form of treatment for
localized blad-der cancer. Use of nonspecific agents in advanced cancer
remainslimited, however, and research is continuing in an effort to iden-tify
other uses and other agents.
Monoclonal Antibodies
Monoclonal antibodies (MoAbs), another type of BRM, became available
through technological advances, enabling investigators to grow and produce
specific antibodies for specific malignant cells. Theoretically, this type of
specificity allows the MoAb to de-stroy the cancer cells and spare normal
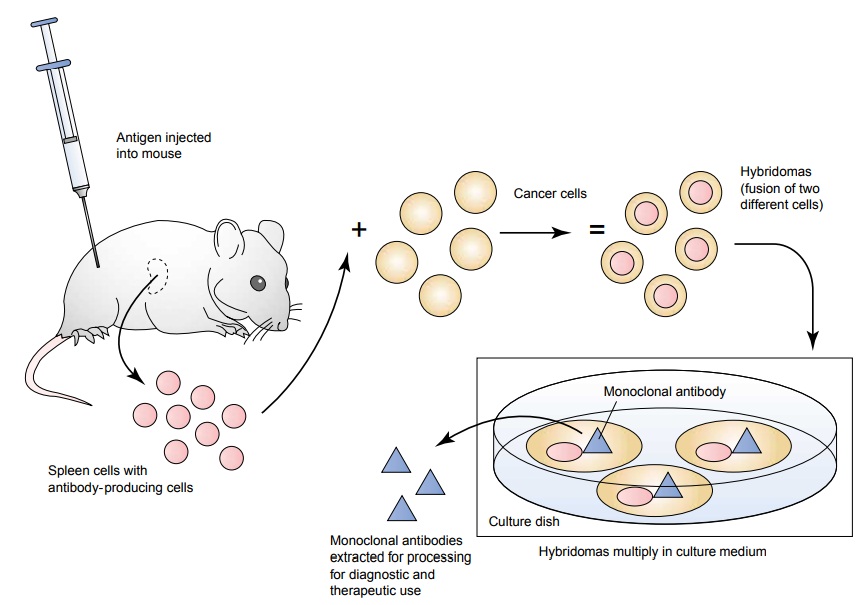
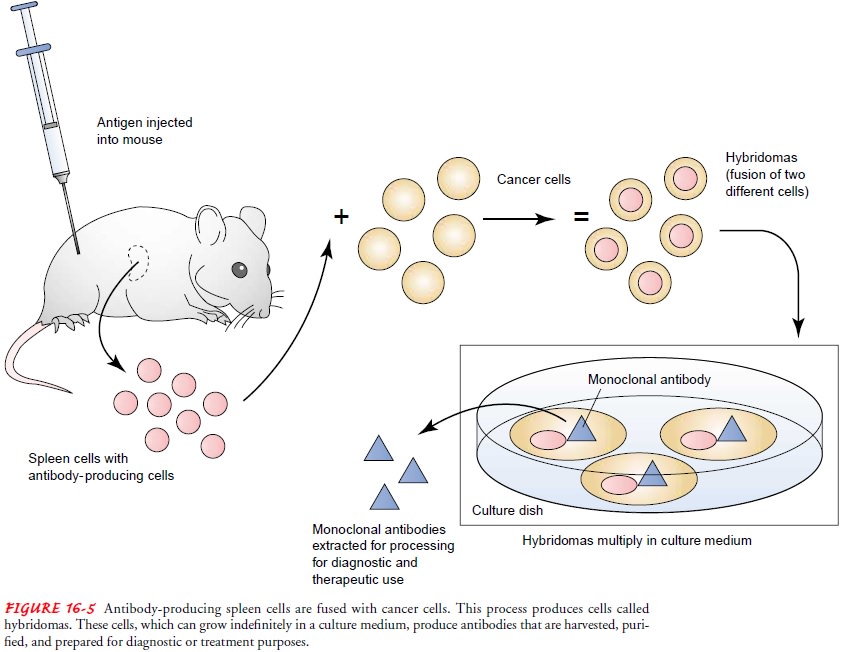
cells. The production of MoAbs involves injecting tumor cells that act as
antigens into mice. Antibodies made in response to injected antigens can be
found in the spleen of the mouse. Antibody-producing spleen cells are combined
with a cancer cell that has the ability to grow indefinitely in culture medium
and continue producing more anti-bodies. The combination of spleen cells and
the cancer cells is re-ferred to as a hybridoma. From hybridomas that continue
to grow in the culture medium, the desired antibodies are harvested, pu-rified,
and prepared for diagnostic or therapeutic use (Fig. 16-5). Alternative methods
of producing MoAbs using human or ge-netically engineered sources are under
investigation.
MoAbs are being used as
aids in diagnostic evaluation. By at-taching a radioactive substance to the
MoAb, physicians can detect both primary and metastatic tumors through
radiologic techniques. This process is referred to as radioimmunodetection. OncoScint
(Cytogen Corp., Princeton, NJ) is a U.S. Food and Drug Admin-istration
(FDA)-approved MoAb that is used to assist in diagnos-ing ovarian and
colorectal cancers. The use of MoAbs in detecting breast, gastric, and prostate
cancers and lymphoma is under inves-tigation. MoAbs are also used in purging
residual tumor cells from the bone marrow or peripheral blood of patients who
are under-going BMT for peripheral stem cell rescue after high-dose cyto-toxic
therapy.
Several MoAbs have been
approved for treatment in cancer. Rituximab (Rituxan) is used for the treatment
of relapsed or re-fractory non-Hodgkin’s lymphoma (Kosits & Callaghan,
2000). Trastuzumab (Herceptin) is approved as a single agent or given in
addition to chemotherapy for the treatment of some types of metastatic breast
cancer (Yarbro, 2000). Alemtuzumab (Cam-path) is used in the treatment of some
forms of leukemia (Seeley
DeMeyer, 2002). Gemtuzumab ozogomicin (Mylotarg) is a combination of a
MoAb and the antitumor antibiotic calichea-micin, which is used for the
treatment of a specific type of acute myeloid leukemia (Sorokin, 2000).
Gemtuzumab ozogomicin is an example of immunoconjugate therapy or a “magic
bullet” that transports cancer-killing substances to the cancer cells.
Ibritumomab-tiuxetan (Zevalin) is another form of immuno-conjugate therapy that
combines a monoclonal antibody and a radioactive source for the treatment of
specific types of non-Hodgkin’s lymphoma. The monoclonal antibody delivers the
radioactive source to the malignant cells, causing the cells to be destroyed by
both radioactivity and normal immune responses (Estes, 2002). Researchers are
continuing to explore the develop-ment and use of other MoAbs either alone or
in combination with other substances such as radioactive materials,
chemothera-peutic agents, toxins, hormones, or other BRMs.
Cytokines
Cytokines, substances produced by cells of the immune system toenhance the
production and functioning of components of the immune system, are also the
focus of cancer treatment research. Cytokines are grouped into families, such
as interferons, interleukins, colony-stimulating factors, and tumor
necrosis factors (TNFs).
INTERFERON
Interferons (IFNs) are examples of cytokines with both antiviral and
antitumor properties. When stimulated, all nucleated cells are capable of
producing these glycoproteins, which are classified according to their biologic
and chemical properties: IFN-α is produced by leukocytes, IFN-β is produced by fibroblasts, and IFN-γ is produced by lymphocytes.
Although the exact antitumor effects of IFNs have not been thoroughly
established, it is thought that they either stimulate the immune system or
assist in preventing tumor growth. The anti-tumor effects are dependent on the
type of IFN and the disease for which IFN is being used. IFNs enhance both
lymphocyte and antibody production. They also facilitate the cytolytic or cell
destruction role of macrophages and natural killer cells. Addi-tionally, IFNs
can inhibit cell multiplication by increasing the duration of various phases of
the cell cycle.
The effects of IFN have been demonstrated in a variety of malignancies.
IFN-α has been approved by
the FDA for treat-ing hairy-cell leukemia, Kaposi’s sarcoma, chronic
myelogenous leukemia, high-grade non-Hodgkin’s lymphoma, and melanoma. Other
positive responses have been seen in hematologic malig-nancies and renal
carcinomas. IFN-α, IFN-β, and IFN-γ have been approved by the FDA for the treatment of several
non-malignant diseases. IFN is administered through subcutaneous,
intramuscular, intravenous, and intracavitary routes. Efforts are underway to
establish the effectiveness of IFN for various malig-nancies in combination
with other treatment regimens.
INTERLEUKINS
Interleukins are a subgroup of cytokines known as lymphokines and
monokines because they are primarily produced by lympho-cytes and monocytes.
About 15 different interleukins have been identified. They act by signaling and
coordinating other cells of the immune system. The FDA has approved
interleukin-2 (IL-2) as a treatment option for renal cell cancer and metastatic
melanoma in adults. Originally referred to as T-cell growth factor, IL-2 is
known to stimulate the production and activation of several dif-ferent types of
lymphocytes. In addition, IL-2 enhances the pro-duction of other types of
cytokines and plays a role in influencing both humoral and cell-mediated immunity.
Clinical trials are beng conducted on IL-2 as well as other
in-terleukins, such as IL-1, IL-4, and IL-6, for their roles in treating other
cancers. Some early-stage clinical trials are assessing the ef-fects of
interleukins in combination with chemotherapy. In ad-dition, interleukins are
being investigated for their role as growth factors for treating
myelosuppression after the use of some forms of chemotherapy.
HEMATOPOIETIC GROWTH FACTORS (COLONY-STIMULATING FACTORS)
Hematopoietic growth
factors, also known as colony-stimulating factors, are hormone-like substances
naturally produced by cells within the immune system. Hematopoietic growth
factors of dif-ferent types regulate the production of all cells in the blood,
in-cluding neutrophils, macrophages, monocytes, red blood cells, and platelets.
FDA approval of GM-CSF, G-CSF, IL-11, and EPO (Epogen) has contributed
significantly to the supportive care of patients with cancer.
Although these agents do not treat the underlying malig-nancy, they do target the effects of myelotoxic cancer therapies (adversely affecting the bone marrow), such as radiation and chemotherapy. Previously, the myelotoxic or bone marrow sup-pressive effects of chemotherapy had imposed limits on some chemotherapy agents and contributed to the development of life-threatening infections.
GM-CSF is used to treat the neutropenia
(decreased numbers of neutrophils in the blood) associated with BMT. G-CSF is
used to treat neutropenia associated with chemotherapy for solid tumor
malignancies. IL-11 is used to prevent severe thrombocy-topenia and reduce the
need for platelet transfusions in patients following myelosuppressive therapy
for nonmyeloid cancers. EPO is used to treat anemia in cancer patients as well
as in pa-tients with chronic renal disease and in patients with HIV infec-tion
with zidovudine-induced anemia. Other growth factors, such as macrophage
colony-stimulating factor and IL-3, are being investigated.
TUMOR NECROSIS FACTOR
TNF is a cytokine naturally produced by macrophages, lympho-cytes,
astrocytes, and microglial cells of the brain. The exact role of TNF is still
under investigation. In vitro studies have shown TNF to stimulate other cells
of the immune response; in animal studies it has been shown to have direct
tumor-killing activity. Clinical trials using systemic TNF have been halted
because of severe toxicities (Pazadur, Coia, Hoskins & Wagman, 2001).
Cur-rent clinical trials are examining local administration of TNF for patients
with sarcomas and melanomas of the extremities.
Retinoids
Retinoids are vitamin A derivatives (retinol, all-trans-retinoic acid, and 13-cis-retinoic
acid) that play a role in growth, repro-duction, epithelial cell
differentiation, and immune function. All-trans-retinoic
acid (tretinoin) has been granted FDA approval fortreating acute promyelocytic
leukemia, a rare form of leukemia. Retinoids are being tested for treating both
hematologic cancers and solid tumors and for preventing a variety of cancers
(Evans & Kaye, 1999; Kelloff, 2000; Kurie, 1999).
Nursing Management in Biologic Response Modifier Therapy
Patients receiving BRM therapy have many of the same needs as cancer
patients undergoing other treatment approaches. How-ever, some BRM therapies
are still investigational and considered a last-chance effort by many patients
who have not responded to standard treatments. Consequently, it is essential
that the nurse assess the need for education, support, and guidance for both
the patient and family and assist in planning and evaluating pa-tient care.
MONITORING THERAPEUTIC AND ADVERSE EFFECTS
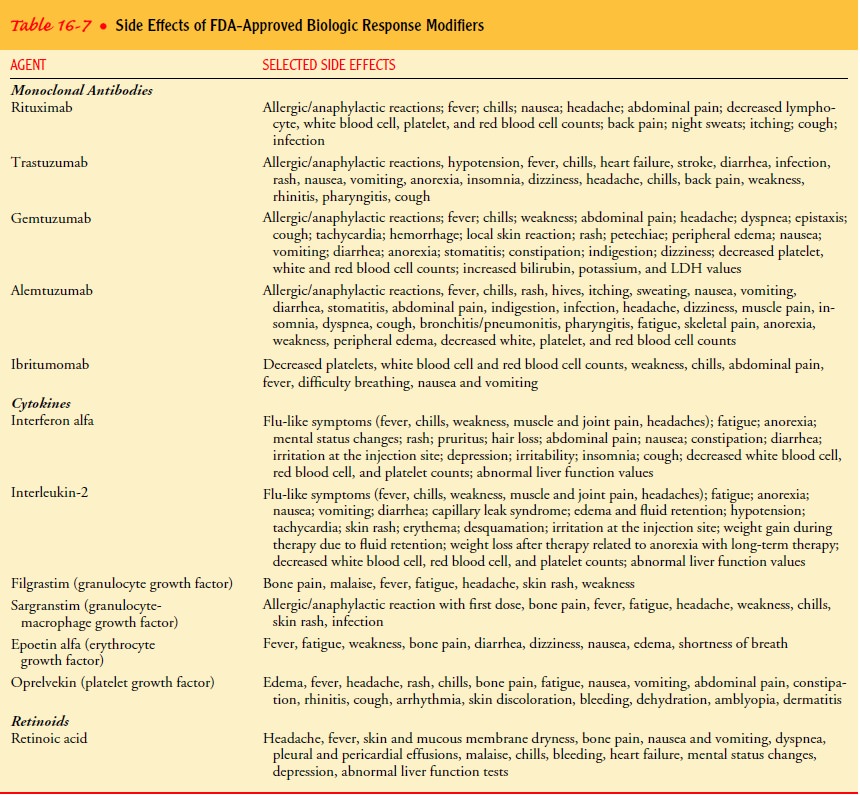
Nurses need to be familiar with each agent given and the poten-tial
effects (Table 16-7). Adverse effects, such as fever, myalgia, nausea, and
vomiting, as seen with IFN therapy, may not be life-threatening. However,
nurses must be aware of the impact of these side effects on the patient’s
quality of life. Other life-threatening adverse effects (eg, capillary leak
syndrome, pulmonary edema, and hypotension) may occur with IL-2 therapy. Nurses
must work closely with physicians to assess and manage potential toxicities of
BRM therapy. Because of the investigational nature of many of these agents, the
nurse will be administering them in a research setting. Accurate observations
and careful documen-tation are essential components of patient assessment and
data collection.
PROMOTING HOME AND COMMUNITY-BASED CARE
Teaching Patients Self-Care.
Some BRMs,
such as IFN, EPO,and G-CSF, can be administered by the patient or family in the
home. Nurses teach patients and families, as needed, how to ad-minister these
agents through subcutaneous injections. Further, they provide instructions
about side effects and assist patients and families to identify strategies to
manage many of the common side effects of BRM therapy, such as fatigue,
anorexia, and flu-like symptoms.
Continuing Care.
Referral for home care is usually indicated tomonitor the patient’s responses to treatment and continue and reinforce teaching. During home visits, the nurse assesses the pa-tient’s and family’s technique in administering medications. The nurse collaborates with physicians, third-party payors, and phar-maceutical companies to help patients obtain reimbursement for home administration of BRM therapies. The nurse also reminds patients about the importance of keeping follow-up appoint-ments with the physician and assesses the patient’s need for changes in care.
Related Topics