Chapter: Basic & Clinical Pharmacology : Opioid Analgesics & Antagonists
Basic Pharmacology of the Opioid Analgesics
BASIC PHARMACOLOGY OF THE OPIOID ANALGESICS
Source
Opium, the source of
morphine, is obtained from the poppy, Papaver
somniferum and P album. After
incision, the poppy seedpod exudes a white substance that turns into a brown
gum that is crude opium. Opium contains many alkaloids, the principal one being
morphine, which is present in a concentration of about 10%. Codeine is
synthesized commercially from morphine.
Classification & Chemistry
Opioid drugs include
full agonists, partial agonists, and antago-nists. Morphine is a full agonist
at the l(mu)-opioid
receptor,
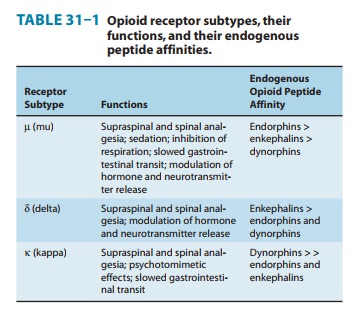
the major analgesic opioid receptor (Table 31–1). In contrast, codeine
functions as a partial (or “weak”) μ-receptor agonist. Other opioid receptor
subtypes include c(delta) and j(kappa) receptors. Simple substitution of an allyl group on the
nitrogen of the full agonist morphine
plus addition of a single hydroxyl group results in naloxone, a strong μ-receptor antagonist. The structures of some of
these compounds are shown later. Some opioids, eg, nalbuphine, are capable of
producing an agonist (or partial agonist) effect at one opioid receptor subtype
and an antagonist effect at another. The receptor activating properties and
affinities of opioid analgesics can be manipulated by pharmaceuti-cal
chemistry; in addition, certain opioid analgesics are modified in the liver,
resulting in compounds with greater analgesic action. Chemically, the opioids
derived from opium are phenanthrene derivatives and include four or more fused
rings, while most of the synthetic opioids are simpler molecules.
Endogenous Opioid Peptides
Opioid alkaloids (eg, morphine) produce analgesia through actions at receptors in the central nervous system (CNS) that respond to certain endogenous peptides with opioid-like pharma-cologic properties. The general term currently used for these endogenous substances is endogenous opioid peptides.
Three families of
endogenous opioid peptides have been described in detail: the endorphins, the pentapeptide enkepha-lins methionine-enkephalin (met-enkephalin) and
leucine-enkephalin (leu-enkephalin),
and the dynorphins. The three
families of opioid receptors have overlapping affinities for these endogenous
peptides (Table 31–1).
The endogenous opioid
peptides are derived from three precursor proteins: prepro-opiomelanocortin
(POMC), preproenkephalin (proenkephalin A), and preprodynorphin (proenkephalin
B). POMC contains the met-enkephalin sequence, β-endorphin, and several nonopioid peptides,
including adrenocorticotropic hormone (ACTH), β-lipotropin, and melanocyte-stimulating
hormone. Preproenkephalin contains six copies of met-enkephalin and one copy of
leu-enkephalin. Leu- and met-enkephalin have slightly higher affinity for the δ (delta) than for the μ-opioid receptor
(Table 31–1). Preprodynorphin yields several active opioid peptides that
contain the leu-enkephalin sequence. These are dynorphin A,dynorphin B, andαandβ
neoendorphins. The
endogenous pep-tides endomorphin-1
and endomorphin-2 also possess many
of the properties of opioid peptides, notably analgesia and high-affinity
binding to the μ
receptor. Endomorphin-1 and -2 selectively activate central and peripheral μ-opioid receptors but
much about them remains unknown, including the identity of their
preproendomor-phin gene. Both the endogenous opioid precursor molecules and the
endomorphins are present at CNS sites that have been implicated in pain modulation.
Evidence suggests that they can be released during stressful conditions such as
pain or the anticipation of pain and diminish the sensation of noxious stimuli.
Whether acupuncture releases endogenous opioid peptides is under investigation.
In contrast to the
analgesic role of leu- and met-enkephalin, an analgesic action of dynorphin
A—through its binding to κ (kappa)-opioid receptors—remains
controversial. Dynorphin A is also found in the dorsal horn of the spinal cord,
where it may play a critical role in the sensitization
of nociceptive neurotransmission. Increased levels of dynorphin can be found in
the dorsal horn aftertissue injury and inflammation. This elevated dynorphin
level is proposed to increase pain and induce a state of long-lasting
hyper-algesia. The pronociceptive action of dynorphin in the spinal cord
appears to be independent of the opioid receptor system but dependent on the
activation of the bradykinin receptor. Moreover, dynorphin A can bind and
activate the N-methyl-D-aspartate
(NMDA)-receptor complex, a site of action that is the focus of intense
therapeutic development.
Recently, a novel
receptor-ligand system homologous to the opioid peptides has been found. The
principal receptor for this system is the G protein-coupled orphanin opioid-receptor-likesubtype 1
(ORL1). Its endogenous ligand has been termednoci-ceptin by one group of investigators andorphanin FQby anothergroup. This ligand-receptor system is
currently known as the N/OFQ system.
Nociceptin is structurally similar to dynorphinexcept for the absence of an
N-terminal tyrosine; it acts only at the ORL1 receptor, now known as NOP. The N/OFQ system is widely
expressed in the CNS and periphery, reflecting its equally diverse biology and
pharmacology. As a result of experiments using highly selective NOP receptor
ligands, the N/OFQ system has been implicated in both pro- and anti-nociceptive
activity as well as in the modulation of drug reward, learning, mood, anxiety,
and cough processes, and of parkinsonism.
Related Topics