Chapter: Basic & Clinical Pharmacology : Opioid Analgesics & Antagonists
MILD to Moderate Agonists
MILD TO MODERATE AGONISTS
Phenanthrenes
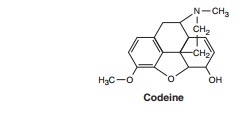
Codeine, dihydrocodeine, and
hydrocodone are all somewhatless efficacious than morphine and often have
adverse effects that limit the maximum tolerated dose when one attempts to
achieve analgesia comparable to that of morphine. Oxycodone is more potent and is prescribed alone in higher doses as
immediate-release or controlled-release forms for the treatment of moderate to
severe pain. Combinations of hydrocodone or oxycodone with acetaminophen are
the predominant formulations of orally administered analgesics for the
treatment of mild to moderate pain. However, there has been a large increase in
the use of con-trolled-release oxycodone at the highest dose range.
Since each controlled-release tablet of oxycodone contains a large quantity of oxycodone to allow for prolonged action, those intent on abusing the old formulation have modified the tablets, achieving high levels instantly, resulting in abuse and possible fatal overdose. The FDA recently approved a new formulation of the controlled-release form of oxycodone that reportedly prevents the tablets from being cut, broken, chewed, crushed, or dissolved to release more oxycodone. This new formulation will hopefully lead to less abuse by snorting or injection. Approximately half a million people used a controlled-release form of oxycodone for the first time in 2008, which prompted the FDA to require the manufac-turer to collect data on abuse or misuse of the drug. The FDA is also requiring a Risk Evaluation and Mitigation Strategy (REMS) that will include the issuance of a medication guide to patients and a requirement for prescriber education regarding the appro-priate use of opioid analgesics in the treatment of pain. (See Box: Educating Opioid Prescribers.)
Phenylheptylamines
Propoxyphene is chemically related to methadone but hasextremely low
analgesic activity. Its low efficacy makes it unsuit-able, even in combination
with aspirin, for severe pain. The increasing incidence of deaths associated
with its use and misuse caused it to be withdrawn in the United States.
Phenylpiperidines
Diphenoxylate and its metabolite, difenoxin, are not used foranalgesia but for the treatment of diarrhea. They are scheduled for minimal control (difenoxin is Schedule IV, diphenoxylate Schedule V; see inside front cover) because the likelihood of their abuse is remote. The poor solubility of the compounds limits their use for parenteral injection. As antidiarrheal drugs, they are used in combination with atropine. The atropine is added in a concentration too low to have a significant antidiarrheal effect but is presumed to further reduce the likelihood of abuse.
Loperamide is a phenylpiperidine derivative used to controldiarrhea.
However, due to action on peripheral μ-opioid receptors and lack of effect on CNS
receptors, there is renewed interest in its potential for the treatment of
neuropathic pain. Its potential for abuse is considered very low because of its
limited access to the brain. It is therefore available without a prescription.The
usual dose with all of these antidiarrheal agents is two tablets to start and
then one tablet after each diarrheal stool.
Educating Opioid Prescribers
The treatment of pain is a difficult clinical-pharmacologic problem, and prescribers of opioids have often failed to appreciate this difficulty. As a result, there have been large increases of drug abuse cases in the USA and a nearly fourfold increase in overdose deaths due to prescription opioids between 1999 and 2009. These statistics have prompted the Food and Drug Administration to formulate plans for opioid manufacturers to provide training for all opioid prescribers. The FDA is working to devise methods by which this training would be mandatory for all prescribers and would emphasize the thorough understanding of opioid clinical pharmacology with special education about long-acting and extended-release formulations. The educational emphasis on the long-acting and sustained-release formulations (eg, methadone, oxycodone) reflects their association with skyrocketing mor-bidity and mortality.
Related Topics