Chapter: Modern Medical Toxicology: Miscellaneous Drugs and Poisons: Gastrointestinal and Endocrinal Drugs
Antimetabolites
Antimetabolites
Folic Acid Antagonists
Methotrexate (MTX)
Folic acid antagonists or
antifolates, hold a special place in anticancer chemotherapy, since they were
the first to produce striking remissions in leukaemia, and the first cure for a
solid tumour (choriocarcinoma). Methotrexate even today remains one of the most
important of the antifolates, and is used in the treatment of lymphoma,
lymphocytic leukaemia, breast cancer, small cell carcinoma, rheumatoid
arthritis, and trophoblastic diseases. Methotrexate or MTX is also used as an
immunosup-pressive in organ transplantation.
Methotrexate (MTX) is rapidly
absorbed orally if admin-istered in small doses. Large doses are incompletely
absorbed, and therefore should be given intravenously. In the latter case, the
drug disappears from plasma in a triphasic fashion. The first phase is a rapid
distributive phase, which is followed by a second phase of renal clearance
(half-life of 2 to 3 hours), and a terminal phase of half-life of 8 to 10
hours. If the terminal phase is unduly prolonged, as in renal failure, there
can be severe toxic effects. Renal excretion occurs through a combina-tion of
glomerular filtration and tubular secretion. Therefore, concomitant
administration of drugs that reduce renal blood flow, or which delay drug excretion,
or are nephrotoxic, can lead to severe myelosuppression.
Adverse effects include nausea,
vomiting, diarrhoea, fever, anaphylaxis, and hepatic necrosis. Chronic use
causes oral and gastrointestinal ulceration (sometimes perforation), bone
marrow depression, hepatotoxicity (cirrhosis), renal toxicity, pulmonary
fibrosis, osteoporosis, conjunctivitis, alopecia, encephalopathy, infertility,
and lymphoma. Intrathecal MTX induces three types of toxic reaction—chemical
arachnoiditis (self-limiting), spinal cord and/or nerve damage (may be
revers-ible or progressively fatal), and encephalopathy, with dementia,
convulsions, coma and death.
Overdose can result in pancytopenia
and severe mucositis.
Mortality from high-dose MTX therapy
is about 6%, and occurs primarily when the patient is not monitored regularly
with MTX levels. Treatment involves gastric lavage if the victim is seen early.
Activated charcoal is not effective. Good urinary output (1 to 3 ml/kg/hr) must
be maintained, and the urine may be alkalinised with sodium bicarbonate.
Folinic acid or leucovorin is the specific antidote for MTX. It reverses bone
marrow and GI toxicity, but unfortunately does not resolve neurotoxicity. An
initial dose of leucovorin estimated to produce the same plasma concentration
as the MTX dose should be given as soon as possible. The toxic threshold for
MTX is reported to be 2 × 10-8 mol/L (i.e. 0.02 mmol/L or 20 nmol/L). The dose of
leucovorin should be repeated every 3 to 6 hours until the MTX level falls
below 1 × 10-8 mol/L. Leucovorin therapy may have to be continued for 12 to 24
doses or longer. In all but the most severe cases of MTX, a leucovorin dose of
100 mg/m2 every 6 hours should be effec-tive. Haemoperfusion and haemodialysis
have been reported to be beneficial in MTX overdose. Carboxypeptidase G2 is a
new agent capable of inactivating MTX by cleaving its terminal glutamate group.
However it can cause hypersen-sitivity reactions because of its bacterial
origin. Granulocyte colony stimulating factor (G -CSF) has been used
successfully in some patients with MTX overdose. The suggested dosage of G-CSF
is 125 mcg/kg/day.
Intrathecal MTX overdose must be
treated as follows:
·
CSF drainage—Drainage of 30 ml CSF by lumbar
puncture within the first 15 minutes after the overdose can remove upto 95% of
the drug. Two hours after the overdose, drainage may remove only about 20% of
MTX.
·
CSF washout—With MTX overdoses of more than 100
mg,
·
CSF drainage must be accompanied by ventriculolumbar
perfusion.
·
IV pentobarbitone and phenytoin for convulsions.
·
Alkalinise urine to promote urinary excretion of MTX.
·
Administration of high doses of leucovorin IV (upto 1000 mg)
may be of benefit.
·
Mannitol for cerebral oedema.
·
Maintain fluid balance.
·
Monitor arterial blood gases.
·
Intubation and mechanical ventilation, if patient is
coma-tose.
Pyrimidine Analogues
5-Fluorouracil (5-FU)
The 5-FU requires enzymatic
conversion to the nucleotide (ribosylation and phosphorylation) in order to
exert its cytotoxic activity. It is generally used to treat patients with
metastatic carcinomas of the breast and GI tract. It is also beneficial in
hepatoma and carcinoma of ovary, cervix, urinary bladder, pros-tate, pancreas,
and oropharyngeal areas. 5-FU is administered parenterally and is subsequently
inactivated by dihydropyrimi-dine dehydrogenase, deficiency of which can lead
to profound toxicity even with conventional doses.
Toxic effects include anorexia,
nausea, stomatitis, diar-rhoea, GI ulceration, shock and death. Chronic adverse
effects include myelosuppression (maximal in 2 weeks), alopecia, dermatitis,
acute cerebellar syndrome and cardiotoxicity.
![]()
Cytarabine (Cytosine arabinoside)
Cytarabine is the most effective
antimetabolite used in the treatment of acute myelocytic leukaemia. It has to
be first “acti-vated” by conversion to the 5/-monophosphate nucleotide which is
catalysed by deoxycytidine kinase. This is then converted to the diphosphate
and triphosphate nucleotides which cause potent inhibition of DNA synthesis in
cells.
Cytarabine is usually given IV or
intrathecally. Less than 10% of the injected dose is excreted unchanged in the
urine, while most appears as the inactive, deaminated product arabi-nosyl
uracil.
Adverse effects include vomiting,
diarrhoea, anaphylaxis, and respiratory distress (high doses). Chronic use can
cause bone marrow depression, conjunctivitis, oral ulceration, hepatic damage,
fever, pulmonary oedema, neurotoxicity and rhabdomyolysis.
Purine Analogues
Mercaptopurine
Mercaptopurine
is an important drug in the treatment of leukaemias, especially acute leukaemia
in children. It also has immunosuppressive activity, but its imidazoyl
derivative azathioprine is more effective in this regard. Mercaptopurine is
usually given orally, though the bioavailability by this route is relatively
low.
Adverse
effects include bone marrow depression, anorexia, nausea, vomiting, jaundice,
hepatic necrosis, pancreatitis, and dermatitis. Overdose results in dizziness,
headache, abdominal pain, hepatotoxicity and death.
6-Thioguanine
Thioguanine
is especially useful in the treatment of acute granulocytic leukaemia when
given along with cytarabine. It is generally administered orally, though
absorption is incomplete and erratic by this route. Toxic effects include bone
marrow depression, GI distress and hepatic damage.
Natural Products
Vinca Alkaloids
The vinca alkaloids are obtained
from the periwinkle plant (Vinca rosea)
(Fig 32.6), which is a type of
myrtle. Important alkaloids include vinblastine, vincristine, vindesine, and
vinorelbine. They are mainly employed in the treatment of lymphomas, Hodgkin’s
disease, acute leukaemias, and certain solid tumours. Only vinorelbine can be
administered orally, while the others are given IV All the vinca alkaloids are
extensively metabolised by the liver, and the metabolites are excreted mainly
in the bile.

Vincristine is more neurotoxic than the other alkaloids, but is much less myelotoxic, the incidence of myelosuppres-sion being only about 5 to 10%. The following are the major adverse effects of vinca alkaloids: leukopenia, anaemia, thrombocytopenia, alopecia, constipation, nausea, vomiting, abdominal pain, haemorrhagic enterocolitis, paraesthesia, peripheral neuritis, hypertension, bronchospasm, sterility, and skin vesiculation.
Occasionally, a syndrome of inappropriate
antidiuretic hormone secretion (SIADH) occurs. Overdose results in fever,
nausea, vomiting, peripheral neuropathy, muscle weakness, convulsions,
hypertension, and bone marrow suppression. Inadvertent intrathecal
administra-tion of vincristine has resulted in ascending paralysis and death.
Vindesine overdosage leads to severe muscle pain, burning sensation in mouth,
tinnitus, diarrhoea, hiccoughs and insomnia.
Treatment involves the administration
of leucovorin which is said to be beneficial in ameliorating peripheral
neuropathy and myelosuppression. However there is no uniform consensus on this.
There have been reports of the utility of glutamic acid, though this matter too
has not yet been clearly resolved. Plasmapheresis was successfully employed in
one case of vincristine overdose. Dialysis is usually ineffective.
Antitumour Antibiotics
Bleomycin
It is obtained from Strep. verticillus and is actually a
mixture of two copper-chelating peptides (bleomycin A 2 and B2).
It is mainly used against squamous carcinomas of the head and neck and lungs,
lymphomas, and testicular tumours. The cytotoxic action results from its
ability to cause fragmentation of DNA. It is usually given parenterally (IM or IV).
Adverse effects include pulmonary
toxicity (interstitial pneumonitis, fibrosis), anaphylactoid reactions,
hyperpyrexia, rash and vesiculation, hyperkeratosis, alopecia, headache and
vomiting.
Dactinomycin (Actinomycin D)
It is also obtained from Streptomyces species, and is mainly used
intravenously in the treatment of rhabdomyosarcoma and Wilms’ tumour in
children. It is also useful in treating Ewing’s tumour and Kaposi’s sarcoma.
Toxic manifestations include
anorexia, nausea, vomiting, haematopoietic suppression with pancytopenia,
proctitis, diar-rhoea, ulcerations of oral mcosa, alopecia and dermal changes.
Mitomycin C
This is obtained from Strep. caespitosus, and is usually given
intravenously in the treatment of carcinoma of colon or stomach.
Adverse effects include
myelosuppression, vomiting, diarrhoea, dermatitis, fever, pulmonary fibrosis.
The most dangerous adverse effect is a haemolytic uraemic syndrome which
results in renal failure. Extravasation of the drug while infusing it can cause
severe local injury.
Daunorubicin, Doxorubicin, and Idarubicin
These are called anthracycline antibiotics and are
produced by the fungus Strep. peucetius
var. Caesius. Idarubicin is actu-ally
a synthetic derivative. Daunorubicin has been useful in the treatment of acute
lymphocytic and granulocytic leukaemias. It is the drug of choice in acute
nonlymphoblastic leukaemia (along with cytarabine). Doxorubicin is effective
not only in the treatment of acute leukaemias and malignant lymphomas, but is
also useful in treating a number of solid tumours.
Toxic effects include
myelosuppression, thrombocytopenia, anaemia, GI disturbances, alopecia,
conjunctivitis, and severe local reactions if extravasation occurs. A serious
adverse effect with all anthracyclines is cardiomyopathy. Cardiac damage may be
minimised by concomitant administration of dexrazoxane,
an iron chelator.
Enzymes
Bleomycin
Escherichia coli produces
two L-asparaginase isozymes, onlyone of which (EC-2) is used as an
antineoplastic agent. The purified E.coli
enzyme is given IV or IM for the treatment of acute lymphoblastic leukaemia and
other lymphoid cancers. Since this enzyme is a foreign protein and causes
hypersen-sitivity reactions in 5 to 20% of patients, other sources have been
made available including Erwinia
chrysanthemi. Also a modified form of the enzyme (PEG-asparaginase) has
been developed, which is obtained by conjugating it with polythylene glycol.
These are much safer.
Toxic
effects include nausea, vomiting, fever with chills, headache, hyperglycaemia,
acute haemorrhagic pancreatitis, renal and hepatic toxicity, and coagulation
defects. CNS toxicity has been reported, characterised by lethargy, stupor and
coma, which is ascribed to a fall in CSF asparagines. “Asparagine rescue”
infusions have been evolved to counter such serious adverse effects.
Epipodophyllotoxins
Podophyllotoxin is an extract of the mandrake or
mayappleplant (Podophyllum peltatum)
(Fig 32.7). Etoposide and temi-poside are
semisynthetic glycosides derived from it. Etoposideis more commonly used and is
given orally or intravenously for the treatment of malignant lymphomas, acute
leukaemias, small cell lung cancer, and some other solid tumours.

Toxic effects include myelosuppression, nausea, vomiting, diarrhoea, alopecia, fever, and allergic reactions. High doses are associated with hepatic damage.
Androgen Inhibitors
Flutamide
Flutamide
is a non-steroidal anti-androgen which is adminis-tered orally for prostatic
cancer. It acts directly on the target tissues either by blocking androgen
uptake or by inhibiting cytoplasmic and nuclear binding of androgen.
Adverse
effects include hot flashes, loss of libido (in about 50% of patients),
impotence, gynaecomastia, nausea, vomiting, diarrhoea, chest pain, blurred
vision, hepatitis, rash, SLE-like syndrome, confusion and depression.
Anti-oestrogens
Tamoxifen
Tamoxifen citrate is an
anti-oestrogen that is effective as pallia-tive treatment for patients with
advanced breast cancer. It is also used as an adjuvant in postmenopausal women
to prevent disease recurrence. Tamoxifen is given orally.
Adverse effects include
hypercalcaemia (in patients with bone metastases), thromboembolic events, hot
flushes, vaginal bleeding, pruritis vulvae, GI upset, vertigo, and an increased
tendency towards endometrial cancer. The last mentioned should be watched for
by conducting yearly pelvic examina-tions, and specific enquiries must be made
regarding pelvic discomfort for vaginal bleeding.
Miscellaneous Agents
Platinum Co-ordination Complexes (Platinoids)
The cytotoxic effects of the
platinum-containing compounds were first discovered in 1965, and since then
many such compounds have been synthesised, of which the important ones include cisplatin, carboplatin, and iproplatin. The platinoids are used
mainly in the treatment of ovarian and testicular tumours, and also cancers of
head and neck, bladder, oesophagus and lung. They are usually given IV.
Common adverse effects include renal
dysfunction,auditory impairment, peripheral neuropathy, and myelosup-pression.
Overdose results in rapid renal failure and death, due to irreversible acute
tubular necrosis. The presence of urinary alanine aminopeptidase and
N-acetyl-beta -D-glucosamidase are early indicators of renal tubular damage.
Renal dysfunction is usually preceded by encephalopathy, convulsions, visual
impairment (negative-type response with electroretinogram), and high-frequency
hearing loss.![]()
Treatment involves the following
measures:
Chloride diuresis promotes the
inactive anionic state of cisplatin and decreases the urine platinum
concentration, which is helpful in nephrotoxicity during therapy.
Hydration with 0.9% sodium chloride,
and an osmotic diuretic (e.g. mannitol) should be administered to achieve a
high urine output (1 to 3 ml/kg/hr), for 6 to 24 hours post-exposure.
Careful assessment of renal function
by regular assays of serum BUN and creatinine, glomerular filtration,
filtration fraction, and renal plasma flow.
Administration of nephroprotectants
post-exposure, e.g. sodium thiosulfate* (IV bolus of 4 gm/m2, followed by
infu-sion of 12 gm/m2 over 6 hours), and diethyldithiocarbamate,** i.e. DDTC (4
gm/m2 as a 1.5 to 3.5-hour infusion). Disulfiram is metabolised to DDTC and can
be used if the latter is not available.
Plasmapheresis is highly beneficial.
Haemodialysis is effec-tive if there is renal failure.
Hydroxyurea
Hydroxyurea
causes cell death by specific inhibition of DNA synthesis, and is administered
orally in the treatment of chronic myeloid leukaemia and some varieties of
solid tumours.
Adverse
effects include bone marrow suppression, nausea, vomiting, diarrhoea,
stomatitis, drowsiness, convulsions, hallu-cinations, alopecia, fever, chills
and renal dysfunction.
Mitoxantrone (Mitozantrone)
Mitoxantrone
is an anthraquinone related chemically to the anthracyclines. It is indicated
in the treament of advanced breast cancer, lymphoma, and acute lymphocytic
leukaemia. It is given IV.
Adverse
effects include myelosuppression, cardiotoxicity, vomiting, alopecia,
stomatitis, fever, and neurological effects. Urine may be discoloured
blue-green. Overdose results in ataxia, nystagmus, loss of vibration sense,
paraesthaesia, convulsions, and hepatic dysfunction. Extravasation causes
tissue necrosis.
Mesna Preparations
Mesna
is usually given as a uroprotectant to prevent haemor-rhagic cystitis resulting
from therapy with cyclophosphamide and ifosfamide. It is usually given
intravenously. Adverse effects include headache, tachycardia, and hypertension.
Overdose can cause vomiting and diarrhoea.
General Treatment Measures for Anti-cancer Drug Overdoses
Stabilisation—
·
Watch for and manage convulsions (if they occur), with IV
diazepam.
·
If there are vital sign abnormalities, establish IV line,
cardiac monitor, oxygen, and assisted ventilation (as needed).
·
Correct abnormalities of ventilation and blood pressure.
·
Arrange for complete haematological analysis (RBC,
haematocrit, WBC, platelets).
·
If patient is asymptomatic even after 12 hours, discharge
can be considered. However, the patient must be subse-quently followed up
weekly with blood counts for at least 4 weeks. Cardiovascular follow-up is
necessary for several months in the case of anthracycline overdose, on account
of frequently delayed onset of cardiotoxicity.
Decontamination—
·
Stomach wash is indicated in oral overdoses. Syrup of ipecac
is not advisable since it may provoke convulsions which are frequently
encountered with antineoplastic drugs.
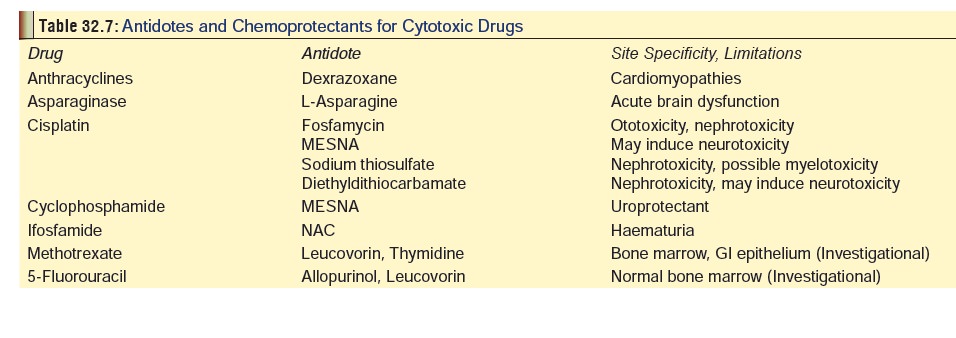
Antidotes—There are very few
antidotes available for antineoplastic drug overdose. Table 32.7 lists some of the accepted antidotal agents.
Elimination Enhancement—While
extracorporeal treat-ment methods such as haemodialysis, haemoperfusion, and
exchange transfusion may help in some cases of overdose, they are generally not
beneficial because most anicancer drugs possess high volumes of distribution,
high protein binding values, and tendency for extensive metabolite formation.
Supportive
Care—
·
Treat convulsions with IV diazepam.
·
Unconscious patients, and those with respiratory diffi-culty
must be intubated.
·
If there is fever (with or without chills), repeated
cultures should be obtained of blood, urine, and sputum. Intravenous
antibiotics may be necessary if there is evidence of infection.
·
Electrolyte depletion should be corrected by replace-ment
therapy with IV electrolytes. This should be accompanied by careful cardiac and
respiratory moni-toring, and periodic arterial blood gas determinations.
·
Oral mucositis necessitates local therapy and parenteral
administration of nutrition. One study indicates that topical application of 1
ml of vitamin E oil (400 mg/ml), twice a day for 5 days is effective for
chemotherapy-induced mucosal lesions.
·
Conjunctivitis can be managed with saline irrigations and
ocular steroids.
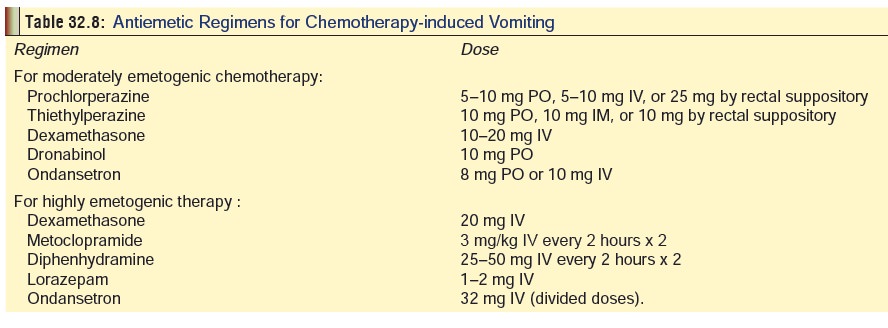
·
Chemotherapy-induced vomiting is the most consistent toxic
effect of almost every anticancer drug, and may be extremely severe and
refractory to treatment. The usual antiemetics employed include phenothiazines
(e.g. prochlorperazine), butyrophenones (e.g. droperidol), and substituted
benzamides (e.g. metoclopramide). Ondansetron is as effective as metoclopramide
in the prevention of chemotherapy-induced vomiting.
·
Antiemetic cocktail: Metoclopramide + Steroids (decadron
or methylprednisolone), + Lorazepam.
·
Table 32.8 outlines common treatment regimens
for chemotherapy-induced nausea and vomiting.
Dehydration must be managed with IV
fluids and moni-toring of CVP.
Nephrotoxicity may require prolonged
dialysis.
Treatment of encephalopathy involves
periodic EEG, lumbar punctures (for CSF analysis), CAT scan, nuclear magnetic
resonance studies, and possible “CSF washout”.
Thrombocytopenia may necessitate
platelet transfusion.
Anaemia and low haematocrit may
necessitate red blood cell transfusions.
Hypertension can be managed with
mannitol or IV sodium nitroprusside.
Related Topics