Chapter: Genetics and Molecular Biology: RNA Polymerase and RNA Initiation
Relating Abortive Initiations to Binding and Initiating
Relating Abortive Initiations to Binding and
Initiating
The binding and initiation reactions explained in
the previous section are described by the following equation:
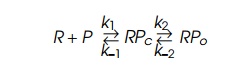
where R is RNA polymerase free in solution, P is uncomplexed promoter RPc is promoter with RNA
polymerase bound in an inactive state definedas “closed,” and RP0 is promoter with RNA
polymerase bound in an active state called “open” because it can immediately
begin transcrip-tion if provided nucleotides.
If RNA
polymerase and DNA containing a promoter are mixed together, then the
concentration of RP0 at
all times thereafter can be calculated in terms of the initial concentrations R and P and the four rate constants; however, the resulting solution is
too complex to be of much use. A reasonably close mathematical description of
the actual situation can be found by making three approximations. The first is
that R be much greater than Po. This is easily
accomplished because theconcentrations of R
and P added are under the
experimentalist’s control. The second is known to enzymologists as the
steady-state assumption. Frequently the rate constants describing reactions of
the type written above are such that, during times of interest, the rate of
change in the amount of RPc,
is small, and the amount of RPc
can be considered to be in equilibrium with R,
P, and RPc. That is,
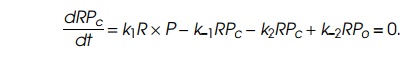
The third
assumption is that k-2 is
much smaller than k2.
Experiments show this to be a very good approximation. RNA polymerase
frequently takes hours or days to dissociate from a promoter. Straightforward
solution of the equations then yields RPo
as a function of time in a useful form:
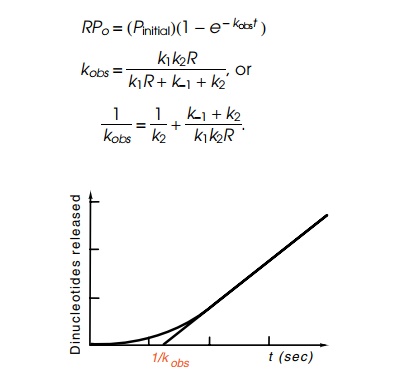
Figure
4.20 Determination of theparameter kobs from the kinetics of incorporation of radioactivity into dinucleotides
After
starting the binding initiation assay, the total amount of oli-gonucleotides
synthesized by any time can be measured by removing a sample from the synthesis
mixture and chromatographically separating nucleoside triphosphates from the
short oligonucleotides. Since the rate of oligonucleotide production is
proportional to RP0, the
total amount of oligonucleotides synthesized as a function of time is given by
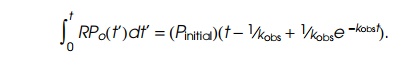
At very
large t this increases linearly as Pinitial(t−1⁄kobs). Hence extrapo-lating the linear
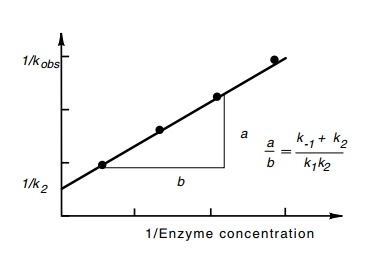
portion of the curve to the point of zero oligonucleotides gives 1/kobs (Fig. 4.20). As seen
above, 1/kobs in the limit
of high R yields 1/k2. At other concentrations,
(k-1 + k2/k1k2)
is a linear function of 1/R.
Performing the abortive initiation reaction at a variety of concentrations of R and measuring the kinetics of
synthesis of oligonucleotides permits straightforward evaluation of k2 (Fig. 4.21). Often, k-1 is much greater than k2, in which case the ratio k1/k-1, called KB,
or equivalently, k-1/k1 which is called Kd, is obtained as well.
A variety
of promoters have been examined by these techniques. Highly active promoters
must bind polymerase well and must perform the initiation “isomerization”
quickly. Less active promoters are poor in binding RNA polymerase or slow in
isomerization.
Related Topics