Chemistry - Number of atoms in a cubic unit cell | 12th Chemistry : UNIT 6 : Solid State
Chapter: 12th Chemistry : UNIT 6 : Solid State
Number of atoms in a cubic unit cell
Number of atoms in a cubic unit cell:
1. Primitive (or) simple cubic unit cell.(SC)
In the simple cubic unit cell, each corner is occupied by an identical atoms or ions or molecules. And they touch along the edges of the cube, do not touch diagonally. The coordination number of each atom is 6.
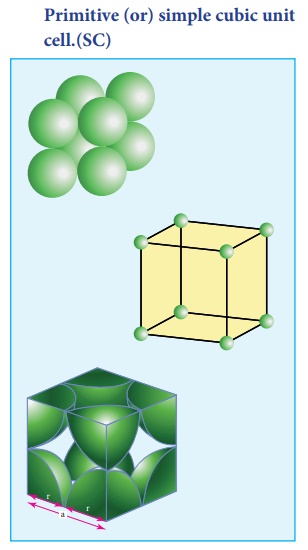
Each atom in the corner of the cubic unit cell is shared by 8 neighboring unit cells and therefore atoms `per unit cell is equal to Nc/8 , where Nc is the number of atoms at the corners.

2. Body centered cubic unit cell. (BCC)
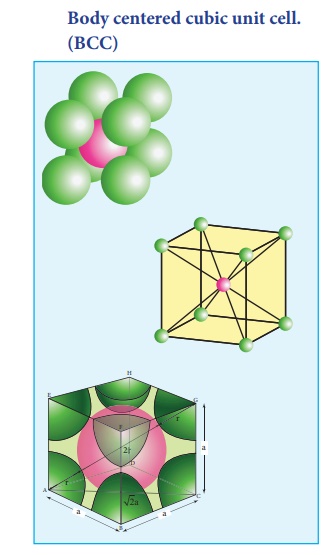
In a body centered cubic unit cell, each corner is occupied by an identical particle and in addition to that one atom occupies the body centre. Those atoms which occupy the corners do not touch each other, however they all touch the one that occupies the body centre. Hence, each atom is surrounded by eight nearest neighbours and coordination number is 8. An atom present at the body centre belongs to only to a particular unit cell i.e unshared by other unit cell.
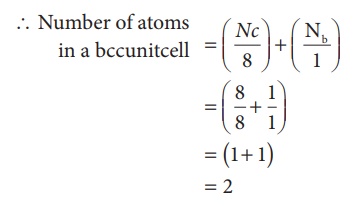
3. Face centered cubic unit cell.(FCC)
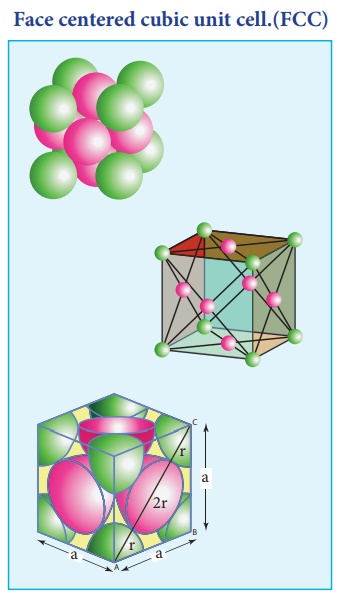
In a face centered cubic unit cell, identical atoms lie at each corner as well as in the centre of each face. Those atoms in the corners touch those in the faces but not each other. The atoms in the face centre is being shared by two unit cells, each atom in the face centers makes ( 1/2 ) contribution to the unit cell.
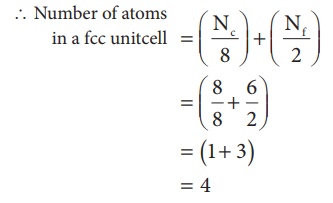
Drawing the crystal lattice on paper is not an easy task. The constituents in a unit cell touch each other and form a three dimensional network. This can be simplified by drawing crystal structure with the help of small circles (spheres) corresponding constituent particles and connecting neighbouring particles using a straight line as shown in the figure.
Related Topics