Chapter: Genetics and Molecular Biology: RNA Polymerase and RNA Initiation
Measurement of Binding and Initiation Rates
Measurement of Binding and Initiation Rates
What experiments can be done to resolve
unambiguously questions about binding and initiation rates? One of the easiest
measurements to perform with RNA polymerase is quantitation of the total RNA
synthe-sized. It is tempting to try to adapt such measurements to the
determi-nation of binding and activation rates of RNA polymerase. The execution
of the experiments and the interpretation of the data are difficult, however,
and many misleading experiments have been done.
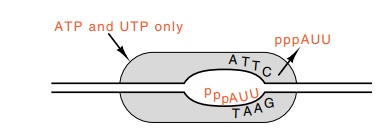
Figure
4.18 Generation of abortive initiation
products by provision of only thefirst two nucleoside triphosphates required by
a promoter.
Somewhat more direct measurements on the initiation
of transcrip-tion are possible. Such measurements were first performed by
McClure, who discovered that as RNA polymerase initiates in vitro it often goes through several cycles of abortive
initiation in which a short two or three nucleotide, or sometimes longer
polynucleotide is synthesized. The sequence of these abortive initiation
products is the same as the 5’ end of the normal RNA transcript. RNA, like DNA,
is elongated in the 5’-to-3’ direction. After one short polynucleotide is made,
RNA polymerase does not come off the DNA, but it remains bound at the promoter,
and another attempt is made at initiation. Additionally, two conditions lead to
the exclusive synthesis of the short polynucleotides: the presence of rifamycin
or the absence of one or more of the ribonucleoside triphos-phates (Fig. 4.18).
If nucleotides are omitted, those present must permit synthesis of a 5’ portion
of the normal transcript. Under these condi-tions, a polymerase molecule bound
to a promoter and in the initiation state will continuously synthesize the
short, abortive initiation polynu-cleotides.
Assay of the short polynucleotides synthesized
under conditions where longer polynucleotides cannot be synthesized provides a
good method of assaying initiation by RNA polymerase on many promoters. The
measurement is free of complexities generated by multiple initia-tions at a
single promoter or of the difficulties in interpretation generated by the
addition of inhibitors. All that is necessary is the omission of the
appropriate nucleoside triphosphates. Once RNA polymerase initiates on a
promoter, it begins production of short polynucleotides, but nothing else
happens at this copy of the promoter.
Let us consider the possibility that the time
required for RNA polym-erase molecules to locate and bind to the promoters is a
significant fraction of the total time required for binding and initiation. An
experi-ment can be performed by mixing RNA polymerase, DNA containing a
promoter, and two or three of the ribonucleoside triphosphates and measuring
the kinetics of appearance of the short polynucleotides that signal initiation.
Suppose that the same experiment is repeated but with the RNA polymerase at
twice the original concentration (Fig. 4.19). In this case the time required
for RNA polymerase to find and bind to the promoter should be half as long as
in the first case, but the time required for the isomerization part of the
initiation process will remain the same.
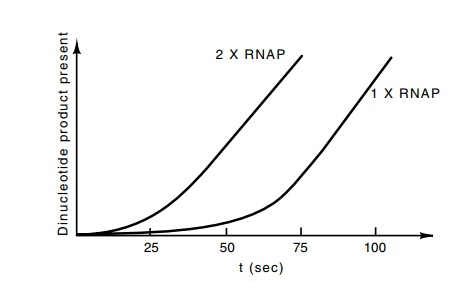
Figure
4.19 The kinetics of synthesis of
dinucleotides following the mixingRNA polymerase and DNA at two polymerase
concentrations differing by a factor of two.
Overall, the synthesis of the short polynucleotides
should begin sooner when polymerase is provided at a higher concentration.
By varying the concentration of polymerase in a set
of experiments and appropriately plotting the results, we can extrapolate to
determine the results that would have been seen if polymerase had been added at
infinite concentration. In such a situation, the delay of polymerase binding to
promoter should be zero. The residual delay in the appearance of
polynucleotides is the time required for isomerization of the polymerase and
DNA to the active state. Knowing this, we can then work back and determine the rate
of binding of RNA polymerase to the promoter.
Consider now the possibility that the initial
binding of RNA polymerase to promoters is fast and that the conversion of the
bound polym-erase to the active state is slow. If only a fraction of the
promoters are occupied by RNA polymerase. Increasing the concentration of RNA
polymerase in this situation will also increase the rate of the initial
appearance of the polynucleotides. The reason is that the initial
concen-tration of polymerase in the bound state increases with increasing
concentrations of polymerase in the reaction. The polynucleotide assay permits
quantitation of the apparent binding constant and of the isomerization rate for
this situation as well. Most importantly, the assay may also be used to
determine how an auxiliary protein assists initiation on some promoters.
Related Topics