Chapter: Medical Surgical Nursing: Fluid and Electrolytes: Balance and Distribution
Calcium Deficit (Hypocalcemia)
CALCIUM
DEFICIT (HYPOCALCEMIA)
Hypocalcemia (lower-than-normal serum concentration of cal-cium) occurs
in a variety of clinical situations. A patient may have a total body calcium
deficit (as in osteoporosis) but a normal serum calcium level. Elderly people
with osteoporosis, who spend an increased amount of time in bed, are at
increased risk for hypo-calcemia as bed rest increases bone resorption.
Several factors can cause hypocalcemia. Primary hypopara-thyroidism
results in this disturbance, as does surgical hypo-parathyroidism. The latter
is far more common. Not only is hypocalcemia associated with thyroid and
parathyroid surgery, but it can also occur after radical neck dissection and is
most likely in the first 24 to 48 hours after surgery. Transient hypo-calcemia
can occur with massive administration of citrated blood (as in exchange
transfusions in newborns), because citrate can combine with ionized calcium and
temporarily remove it from the circulation.
Inflammation of the
pancreas causes the breakdown of pro-teins and lipids. It is thought that
calcium ions combine with the fatty acids released by lipolysis, forming soaps.
As a result of this process, hypocalcemia occurs and is common in pancreatitis.
It has also been suggested that hypocalcemia might be related to ex-cessive secretion
of glucagon from the inflamed pancreas, result-ing in increased secretion of
calcitonin (a hormone that lowers serum calcium).
Hypocalcemia is common
in patients with renal failure be-cause these patients frequently have elevated
serum phosphate levels. Hyperphosphatemia usually causes a reciprocal drop in
the serum calcium level. Other causes of hypocalcemia include inadequate
vitamin D consumption, magnesium deficiency, me-dullary thyroid carcinoma, low
serum albumin levels, alkalosis, and alcohol abuse. Medications predisposing to
hypocalcemia in-clude aluminum-containing antacids, aminoglycosides, caffeine,
cisplatin, corticosteroids, mithramycin, phosphates, isoniazid, and loop
diuretics.
Osteoporosis is associated with prolonged low intake of cal-cium and
represents a total body calcium deficit, even though serum calcium levels are
usually normal. This disorder occurs in millions of Americans and is most
common in postmenopausal women. It is characterized by loss of bone mass,
causing bones to become porous and brittle and therefore susceptible to
fracture.
Clinical Manifestations
Tetany is the most characteristic manifestation of hypocalcemia and hypomagnesemia. Tetany refers to the entire symptom com-plex induced by increased neural excitability. These symptoms are due to spontaneous discharges of both sensory and motor fibers in peripheral nerves. Sensations of tingling may occur in the tips of the fingers, around the mouth, and less commonly in the feet. Spasms of the muscles of the extremities and face may occur. Pain may develop as a result of these spasms.
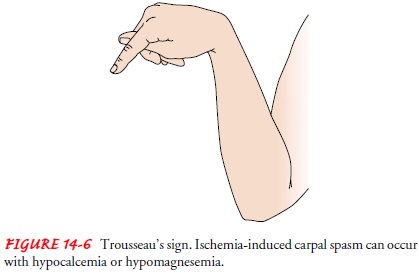
TrousseauŌĆÖs sign (Fig. 14-6) can be elicited by inflating a blood
pressure cuff on the upper arm to about 20 mm Hg above systolic pressure;
within 2 to 5 minutes, carpopedal spasm (an adducted thumb, flexed wrist and
metacarpophalangeal joints, extended in-terphalangeal joints with fingers
together) will occur as ischemia of the ulnar nerve develops. ChvostekŌĆÖs sign
consists of twitching of muscles supplied by the facial nerve when the nerve is
tapped about 2 cm anterior to the earlobe, just below the zygomatic arch.
Seizures may occur because hypocalcemia increases irritability of the
central nervous system as well as of the peripheral nerves. Other changes associated
with hypocalcemia include mental changes such as depression, impaired memory,
confusion, delir-ium, and even hallucinations. A prolonged QT interval is seen
on the ECG due to prolongation of the ST segment; a form of ven-tricular
tachycardia called torsades de pointes may occur.
Assessment and Diagnostic Findings
When evaluating serum calcium levels, one must consider several other
variables, such as the serum albumin level and arterial pH. Because
abnormalities in serum albumin levels may affect inter-pretation of the serum
calcium level, it may be necessary to cal-culate the corrected serum calcium if
the serum albumin level is abnormal. For every decrease in serum albumin of 1
g/dL below 4 g/dL, the total serum calcium level is underestimated by
ap-proximately 0.8 mg/dL. The following is a quick method to cal-culate the
corrected serum calcium level:

An example of the
calculations needed to obtain the corrected total serum calcium level is as
follows:
A patientŌĆÖs reported
serum albumin level is 2.5 g/dL; the re-ported serum calcium level is 10.5
mg/dL.
The decrease in serum
albumin level from normal level (difference from normal albumin of 4 g/dL) is
calculated: 4 g/dL ŌłÆ 2.5 g/dL = 1.5 g/dL
The following ratio is
calculated:
0.8 mg/dL: 1 g/dL =
?mg/dL: 1.5 mg/dL
= 0.8 mg ├Ś 1.5
= 1.2 mg/dL calcium
Add 1.2 to 10.5 mg
(reported serum calcium level) to ob-tain the corrected total serum calcium
level of 11.7 mg/dL. 1.2 + 10.5 mg = 11.7
mg/dL
Clinicians often ignore a low serum calcium level in the pres-ence of a
similarly low serum albumin level. The ionized cal-cium level is usually normal
in patients with reduced total serum calcium levels and concomitant
hypoalbuminemia. When the arterial pH increases (alkalosis), more calcium becomes
bound to protein. As a result, the ionized portion decreases. Symptoms of
hypocalcemia may occur with alkalosis. Acidosis (low pH) has the opposite
effectŌĆöthat is, less calcium is bound to protein and thus more exists in the
ionized form. However, relatively small changes in serum calcium levels occur
in these acidŌĆōbase abnormalities.
Ideally, the laboratory should measure the ionized level of cal-cium. In
many laboratories, however, only the total calcium level is reported; thus,
concentration of the ionized fraction must be estimated by simultaneous
measurement of the serum albumin level. PTH levels are decreased in
hypoparathyroidism. Magne-sium and phosphorus levels need to be assessed to
identify possible causes of decreased calcium.
Medical Management
Acute symptomatic
hypocalcemia is life-threatening and re-quires prompt treatment with IV
administration of calcium (Marx, 2000). Parenteral calcium salts include
calcium gluco-nate, calcium chloride, and calcium gluceptate. Although cal-cium
chloride produces a significantly higher ionized calcium level than calcium
gluconate, it is not used as often because it is more irritating and can cause
sloughing of tissue if it infiltrates. Too-rapid IV administration of calcium
can cause cardiac arrest, preceded by bradycardia. IV calcium administration is
particu-larly dangerous in patients receiving digitalis-derived medica-tions
because calcium ions exert an effect similar to that of digitalis and can cause
digitalis toxicity, with adverse cardiac ef-fects. IV calcium should be diluted
in D5W and given as a slow IV
bolus or a slow IV infusion using a volumetric infusion pump. The IV site must
be observed often for any evidence of infiltra-tion because of the risk for
sloughing of tissues with calcium in-fusions. A 0.9% sodium chloride solution
should not be used with calcium because it will increase renal calcium loss.
Solu-tions containing phosphates or bicarbonate should not be used with calcium
because they will cause precipitation when cal-cium is added. The nurse must
clarify with the physician which calcium salt to administer, because calcium
gluconate yields 4.5 mEq of calcium and calcium chloride provides 13.6 mEq of
calcium. Calcium can cause postural hypotension; therefore, the patient is kept
in bed for IV replacement and blood pressure is monitored.
Vitamin D therapy may be
instituted to increase calcium absorption from the GI tract. Aluminum
hydroxide, calcium acetate, or calcium carbonate antacids may be prescribed to
decrease elevated phosphorus levels before treating hypocal-cemia for the
patient with chronic renal failure. Increasing the dietary intake of calcium to
at least 1,000 to 1,500 mg/day in the adult is recommended (eg, milk products;
green, leafy veg-etables; canned salmon; sardines; fresh oysters). Because
hypo-magnesemia can also cause tetany, if the tetany responds to IV calcium,
then a low magnesium level is explored as a possible cause in chronic renal
failure.
Nursing Management
It is important to
observe for hypocalcemia in patients at risk. Seizure precautions are initiated
when hypocalcemia is severe. The status of the airway is closely monitored
because laryngeal stridor can occur. Safety precautions are taken, as
indicated, if confusion is present.
People at high risk for osteoporosis are instructed about the need for
adequate dietary calcium intake; if not consumed in the diet, calcium
supplements should be considered. Also, the value of regular weight-bearing
exercise in decreasing bone loss should be emphasized, as should the effect of
medications on calcium bal-ance. For example, alcohol and caffeine in high
doses inhibit cal-cium absorption, and moderate cigarette smoking increases
urinary calcium excretion. Additional teaching topics may involve discus-sion
of medications such as alendronate (Fosamax), risedronate (Actonel), raloxifene
(Evista), and calcitonin to reduce the rate of bone loss. Teaching also
addresses strategies to reduce risk for falls.
Related Topics