Chapter: 12th Zoology : Chapter 10 : Applications of biotechnology
Applications of biotechnology in Medicine
Applications in
Medicine
1. Recombinant Human Insulin
The Human insulin is synthesized by the β cells
of Islets of Langerhans in the pancreas. It is formed of 51 aminoacids which
are arranged in two polypeptide chains, A and B. The polypeptide chain A has 21
amino acids while the polypeptide chain B has 30 amino acids. Both A and B
chains are attached together by disulphide bonds. Insulin controls the levels
of glucose in blood. It facilitates the cellular uptake and utilization of
glucose for the release of energy. Deficiency of insulin leads to diabetes
mellitus which is characterized by increased blood glucose concentration and a
complex of symptoms which may lead to death, if untreated. A continuous program
of insulin dependence is required to treat this deficiency.
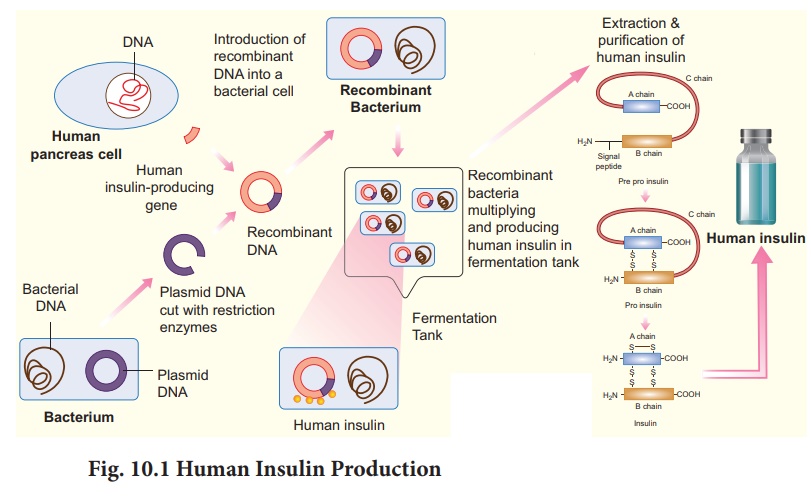
In the early years, insulin isolated and
purified from the pancreas of pigs and cows was used to treat diabetic
patients. Due to minor differences in the structure of the animal insulin as
compared to human insulin, it resulted in the occurrence of allergic reactions
in some diabetic patients. Production of insulin by recombinant DNA technology
started in the late 1970s. This technique involved the insertion of human
insulin gene on the plasmids of E.coli. The polypeptide chains are synthesized
as a precursor called pre-pro insulin, which contains A and B segments linked
by a third chain (C) and preceded by a leader sequence. The leader sequence is
removed after translation and the C chain is excised, leaving the A and B
polypeptide chains (Fig. 10.1).
Insulin was the first ever pharmaceutical
product of recombinant DNA technology administered to humans. The approval to
use recombinant insulin for diabetes mellitus was given in 1982. In 1986 human
insulin was marketed under the trade name Humulin.
Best and Banting in 1921, isolated insulin from the pancreatic islets of a dog and demonstrated its effectiveness against diabetes.
2. Human alpha lactalbumin
Alpha lactalbumin is a protein composed of 123
amino acids and 4 disulphide bridges, with a molecular weight of 14178 Da. In
human milk, α lactalbumin is the most abundant protein comprising 25% of total
protein found in human milk. It is synthesized by the mammary glands. α
lactalbumin binds calcium and zinc ions and possesses bactericidal and anti
tumour activities.
Improvement of the nutritional value of cow’s
milk with transgenic expression of recombinant human alpha lactalbumin has been
attempted. Healthy transgenic cows were produced by somatic nuclear transfer,
in which expression of upto 1.55g/L of recombinant human alpha lactalbumin was
achieved. Similarly transgenic goats were also produced, in which the
expression of Human alpha lactalbumin was about 0.1 to 0.9mg/mL.
Somatic cell nuclear transfer is a technique
for creating a viable embryo from a body cell and an egg cell. This technique
is discussed later in animal cloning.
3. Human Growth Hormone (hGH
At about the same time when recombinant insulin
was first made in E. coli, other research groups worked on human growth
hormones somatostatin and somatotropin. These are peptide hormones secreted by
the pituitary gland that helps in the growth and development by increasing the
uptake of amino acids and promoting protein synthesis. Deficiency of human
growth hormone causes dwarfism, which could be treated by injecting hGH
extracted from the human pituitary glands.
Using recombinant DNA technology hGH can be
produced (Fig. 10.2). The gene for hGH is isolated from the human
pituitary gland cells.
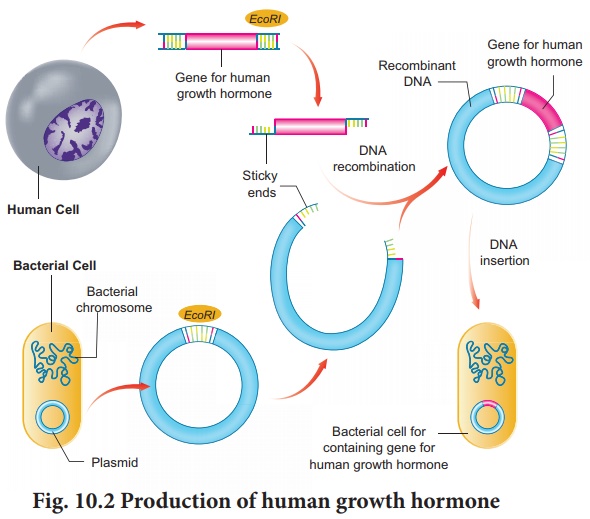
The isolated gene is inserted into a plasmid
vector and then is transferred into E. coli. The recombinant E. coli
then starts producing human growth hormone.The recombinant E. coli are isolated
from the culture and mass production of hGH is carried out by fermentation
technology.
A recombinant form of human growth hormone
called somatropin is used as a drug to treat growth disorders in children.
4. Human Blood-Clotting Factor VIII
You would have studied in your earlier class
that many factors are required for normal blood clotting process and the factor
VIII is one of them. The genes for the formation of factor VIII is located in
the X chromosome. A genetic defect in the synthesis of factor VIII results in
Haemophilia A, a sex-linked disease characterized by prolonged clotting time
and internal bleeding (Refer Chapter 4). Clotting factor VIII isolated from
blood of normal human being was used in the treatment of Haemophilia A.
Requirement of large quantities of blood for this purpose and the risk of
transmission of infectious diseases like AIDS is a disadvantage. Recombinant
DNA technology was used to produce Recombinant Factor VIII in the Chinese
Hamster ovary and in the baby Hamster kidney cells. More recently a cell line
of human origin has been used for the first time to produce human blood
clotting factor VIII.
5. Interferons
Interferons are proteinaceous, antiviral,
species specific substances produced by mammalian cells when infected with
viruses. Interferons were discovered by Alick Isaacs and Jean Lindemann in
1957. Based on the structure of interferons they are classified as α, β and γ interferons.
They stimulate the cellular DNA to produce antiviral enzymes which inhibit
viral replication and protect the cells. Similar to factor VIII , interferons
could be isolated from blood, but the amount of blood required for isolation of
interferons is enormous and not practical. To overcome this issue interferons
could be produced by rDNA technology. The yeast Saccharomyces cerevisiae
is more suitable for production of recombinant interferons than E.coli,
since E.coli does not possess the machinery for glycosylation of proteins.
Interferons are used for the treatment of various diseases like cancer, AIDS,
multiple sclerosis, hepatitis C and herpes zoster. In spite of the therapeutic
applications interferons are not within the reach of the common man due to high
cost for its production.
6. Recombinant Vaccines
Recombinant DNA technology has been used to
produce new generation vaccines.The limitations of traditional vaccine
production could be overcome by this approach.
The recombinant vaccines are generally of
uniform quality and produce less side effects as compared to the vaccines
produced by conventional methods Different types of recombinant vaccines
include subunit recombinant vaccines, attenuated recombinant vaccines and DNA
vaccines.
Subunit recombinant vaccines
Vaccines that use components of a pathogenic
organism rather than the whole organism are called subunit vaccines;
recombinant DNA technology is very suited for developing new subunit
vaccines. It includes components like proteins, peptides and DNAs of pathogenic
organisms. The advantages of these vaccines include their purity in
preparation, stability and safe use.
Attenuated recombinant vaccines
This includes genetically modified pathogenic
organisms (bacteria or viruses) that are made nonpathogenic and are used as
vaccines. It is now possible to genetically engineer the organisms (bacteria or
viruses) and use them as live vaccines and such vaccines are referred to as
attenuated recombinant vaccines.
Edible vaccines are prepared by molecular pharming using the science of genetic engineering. Selected genes are introduced into plants and the transgenic plants are induced to manufacture the encoded protein. Edible vaccines are mucosal targeted vaccines which cause stimulation of both systemic and mucosal immune response. At present edible vaccines are produced for human and animal diseases like measles, cholera, foot and mouth disease and hepatitis.
DNA Vaccines
Genetic immunisation by using DNA vaccines is a
novel approach that came into being in 1990. The immune response of the body is
stimulated by a DNA molecule. A DNA vaccine consists of a gene encoding an
antigenic protein,1inserted onto a plasmid, and then incorporated into the
cells in a target animal. DNA instructs the cells to make antigenic molecules
which are displayed on its surfaces. This would evoke an antibody response to
the free floating antigen secreted by the cells. The DNA vaccine cannot cause
the disease as it contains only copies of a few of its genes. DNA vaccines are
relatively easy and inexpensive to design and produce.
Vaccines produced by these new techniques have
definite advantages like producing target proteins, long lasting immunity and
trigger immune response only against specific pathogens with less toxic
effects.
Recombinant hepatitis B vaccine as a subunit
vaccine is produced by cloning hepatitis B surface antigen (HbsAg) gene inmthe
yeast, Saccharomyces cerevisiae (Fig. 10.3).
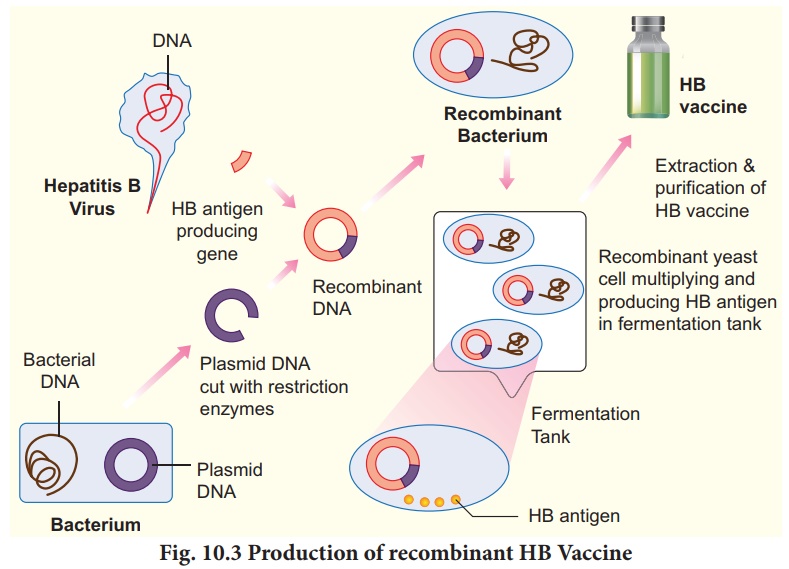
The recombinant vaccine for hepatitis B
(HbsAg) was the first synthetic vaccine launched in 1997 which was marketed by
trade names Recombivax and Engerix-B. India is the fourth country in the world
after USA, France and Belgium to develop an indigenous hepatitis B vaccine.
Related Topics