Chapter: Genetics and Molecular Biology: Protein Structure
Alpha Helix, Beta Sheet, and Beta Turn - Protein Structure
The Alpha Helix, Beta Sheet, and Beta Turn
The existence of the alpha helix was predicted by
Pauling and Cory from careful structural studies of amino acids and peptide
bonds. This pre-diction came before identification of the alpha helix in X-ray
diffraction patterns of proteins. Even though the data were all there, it was
over-looked. The alpha helix is found in most proteins and is a fundamental
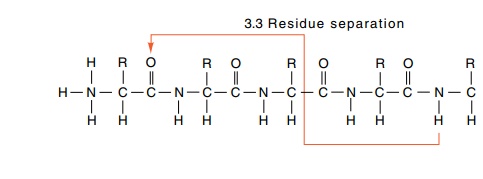
structural element. In the alpha helix, hydrogen bonds are formed between the
carbonyl oxygen of one peptide bond and the amide hydrogen of the amino acid
located three and a third amino acids away.
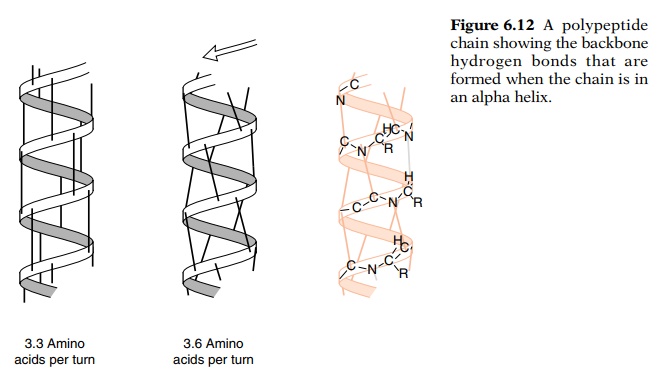
The side chains of the amino acids extend outward
from the helix, and the hydrogen bonds are nearly parallel to the helix axis
(Fig. 6.12). If they were precisely parallel to the axis, the helix pitch would
be 3.33 amino acids per turn, but due to steric constraints, the hydrogen bonds
are somewhat skewed, and the average pitch is found to be 3.6 to 3.7 amino
acids per turn.
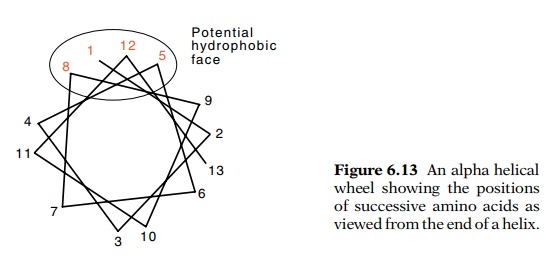
If we look down the axis of an alpha helix, we see
the amino acids winding around in a circle. Every third and then every fourth
amino acid lies on one side of the helix (Fig. 6.13). This pattern follows from
the fact that the alpha helix is nearly 3.5 amino acids per turn. If every
third and then every fourth amino acid were hydrophobic, two such helices could
bind together through their parallel strips of hydrophobic amino acids. This
occurs in structures called coiled coils. These are found in structural
proteins like myosin as well as in a class of transcrip-tional regulators that
dimerize by these interactions. These activators are called leucine-zipper
proteins. They possess leucine residues seven amino acids apart. Strips of
hydrophobic amino acids along one face of alpha helices are frequently found in
bundles containing two, three, or four alpha helices.
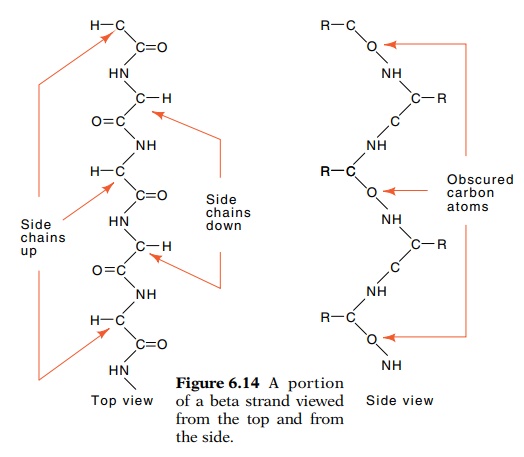
The beta-strand is a second important structural element of proteins. In it the polypeptide chains are quite extended (Fig. 6.14). From a top view the peptide backbone is relatively straight,
but in a side view the peptide backbone is pleated. The side chains of the
amino acids are relatively unconstrained since alternate groups are directed
straight up and straight down. The amide hydrogens and the carboxyl groups are
directed to either side and are available for hydrogen bonding to another
beta-strand lying alongside to form a beta sheet. This second strand can be
oriented either parallel or antiparallel to the first.
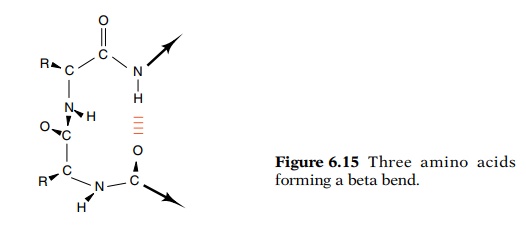
The third readily identified secondary structural
element is the re-verse or beta bend (Fig. 6.15). A polypeptide chain must
reverse direc-tion many times in a typical globular protein. The beta bend is
an energy-effective method of accomplishing this goal. Three amino acids often
are involved in a reverse bend.
Related Topics