Chapter: Biochemistry: Nucleic Acids: How Structure Conveys Information
What is the role of transfer RNA in protein synthesis?
What is the role of transfer RNA
in protein synthesis?
The
smallest of the three important kinds of RNA is tRNA. Different types of tRNA
molecules can be found in every living cell because at least one tRNA bonds
specifically to each of the amino acids that commonly occur in proteins.
Frequently
there are several tRNA molecules for each amino acid. A tRNA is a
single-stranded polynucleotide chain, between 73 and 94 nucleotide residues
long, that generally has a molecular mass of about 25,000 Da. (Note that
bio-chemists tend to call the unit of atomic mass the dalton, abbreviated Da.)
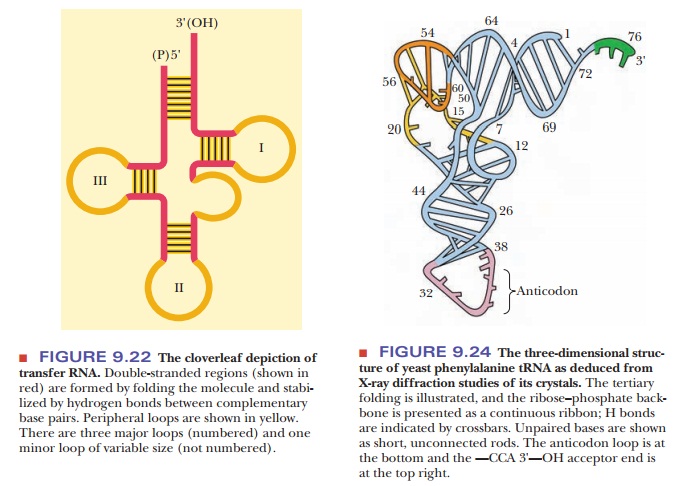
Intrachain hydrogen bonding occurs in tRNA, forming A–U and G–C base pairs similar to those that occur in DNA except for the substitution of uracil for thymine. The duplexes thus formed have the A-helical form, rather than the B-helical form, which is the predominant form in DNA. The molecule can be drawn as a cloverleaf structure, which can be considered the secondary structure of tRNA because it shows the hydrogen bonding between certain bases (Figure 9.22).
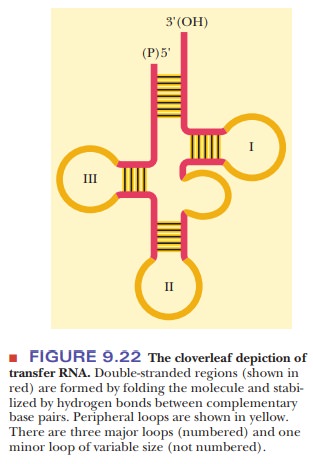
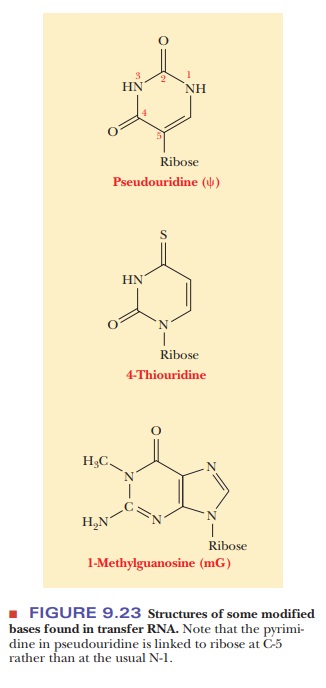
The hydrogen-bonded portions of the molecule are called stems, and the non-hydrogen-bonded
portions are loops. Some of these
loops contain modified bases (Figure 9.23). During protein synthesis, both tRNA
and mRNA are bound to the ribosome in a definite spatial arrange-ment that
ultimately ensures the correct order of the amino acids in the grow-ing
polypeptide chain.
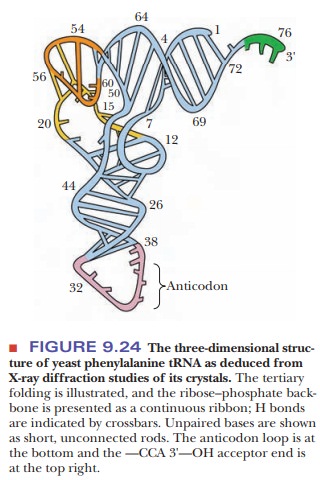
A
particular tertiary structure is necessary for tRNA to interact with the enzyme
that covalently attaches the amino acid to the 2' or 3' end. To produce this
tertiary structure, the tRNA folds into an L-shaped conformation that has been
determined by X-ray diffraction (Figure 9.24).
Related Topics