Chapter: Biochemistry: Nucleic Acids: How Structure Conveys Information
Denaturation of DNA
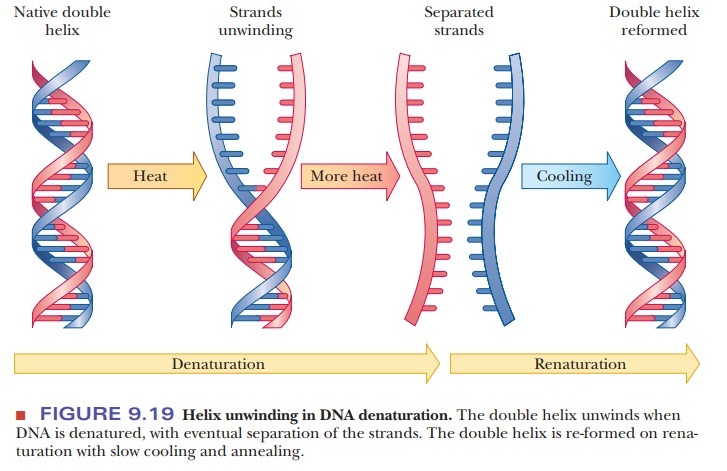
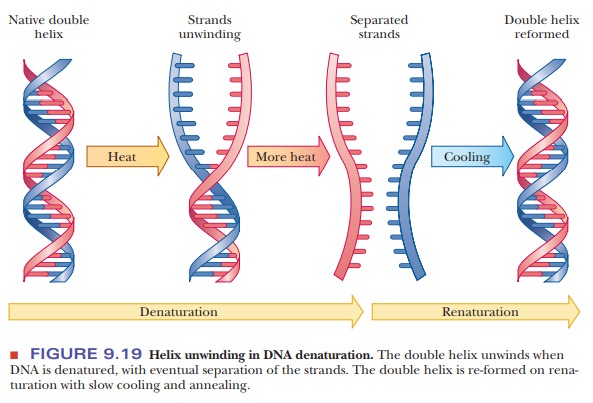
Denaturation of DNA
We have
already seen that the hydrogen bonds between base pairs are an important factor
in holding the double helix together. The amount of stabilizing energy
associated with the hydrogen bonds is not great, but the hydrogen bonds hold
the two polynucleotide chains in the proper alignment. However, the stacking of
the bases in the native conformation of DNA contributes the largest part of the
stabilization energy. Energy must be added to a sample of DNA to break the
hydrogen bonds and to disrupt the stacking interactions. This is usually
carried out by heating the DNA in solution.
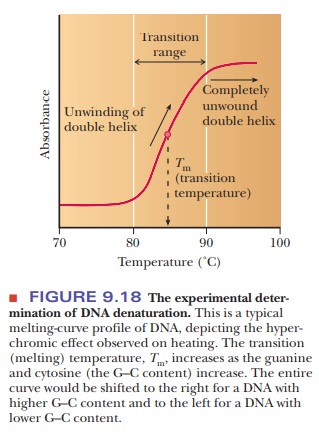
How can we monitor DNA denaturation?
The heat
denaturation of DNA, also called melting,
can be monitored experimentally by observing the absorption of ultraviolet
light. The bases absorb light in the 260-nm-wavelength region. As the DNA is
heated and the strands separate, the wavelength of absorption does not change,
but the amount of light absorbed increases (Figure 9.18). This effect is called
hyperchromicity. It is based on the
fact that the bases, which are stacked on top of one another in native DNA,
become unstacked as the DNA is denatured.
Because
the bases interact differently in the stacked and unstacked orienta-tions,
their absorbance changes. Heat denaturation is a way to obtain single-stranded
DNA (Figure 9.19), which has many uses. When DNA is replicated, it first
becomes single-stranded so that the complementary bases can be aligned. This
same principle is seen during a chemical reaction used to determine the DNA
sequence . A most ambitious example of this reaction is described in the
following Biochemical Connections box.
Under a
given set of conditions, there is a characteristic midpoint of the melting
curve (the transition temperature, or melting temperature, written Tm) for
DNA from each distinct source. The underlying reason for this prop-erty is that
each type of DNA has a given, well-defined base composition. A G–C base pair
has three hydrogen bonds, and an A–T base pair has only two. The higher the
percentage of G–C base pairs, the higher the melting temperature of a DNA
molecule. In addition to the effect of the base pairs, G–C pairs are more
hydrophobic than A–T pairs, so they stack better, which also affects the
melting curve.
Renaturation
of denatured DNA is possible on slow cooling (Figure 9.18). The separated
strands can recombine and form the same base pairs responsible for maintaining
the double helix.
Summary
The two strands of the double helix can be
separated by heating DNA samples. This process is called denaturation.
DNA denaturation can be monitored by observing
the rise in ultraviolet absorption than accompanies the process.
The temperature at which DNA
becomes denatured by heat depends on its base composition; higher temperatures
are needed to denature DNA rich in G–C base pairs.
Related Topics