Chapter: Biotechnology Applying the Genetic Revolution: Proteomics
Western Blotting of Proteins
WESTERN
BLOTTING OF PROTEINS
Often researchers will use
Western blotting to identify proteins. Western blots rely on having an antibody
to the protein. Antibodies are extremely specific and will bind only to one
target protein.
The first step is to separate
the proteins by size by either standard SDS-PAGE or 2D-PAGE. The proteins are
then transferred from the gel to a type of paper membrane made of
nitrocellulose. Other types of membranes are made from nylon and are stronger.
Either way, the membrane must have a positive charge so that the negatively
charged proteins will stick to its surface. The proteins are moved from the gel
to the membrane with an electric current as shown in Fig. 9.4.
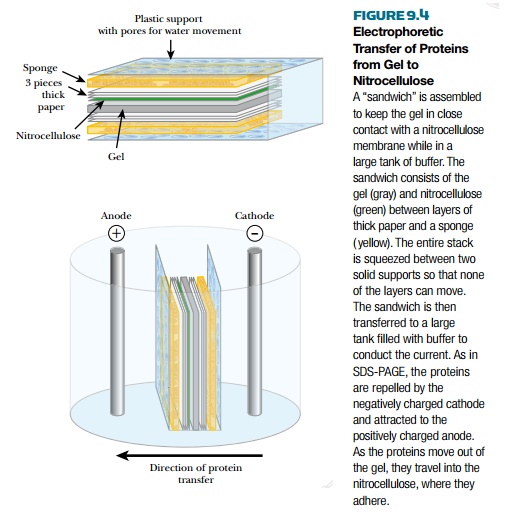
After the proteins are
attached to the nitrocellulose membrane, the primary antibody is used to find
the location of the target protein. However, many areas of the membrane will
not have any protein bound, because the corresponding area of the protein gel
was empty. These blank areas are positively charged and can bind
nonspecifically to the antibody. Therefore, these sites must be blocked. Often,
the membranes are soaked in reconstituted nonfat dry milk. The milk proteins
mask the unused sites on the membrane and will not bind to the antibody. Next,
the antibody is added to the membrane in a solution. The antibody will bind
only to its target protein, and nowhere else on the membrane, because it
recognizes only one specific epitope of its target protein.
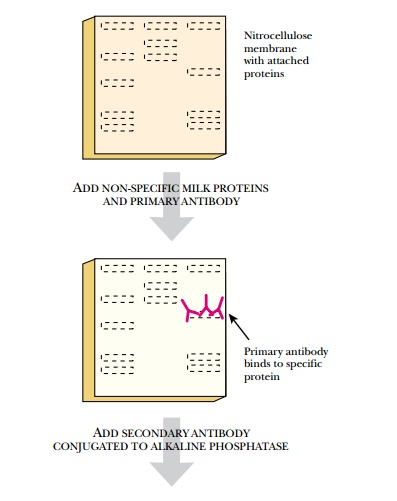
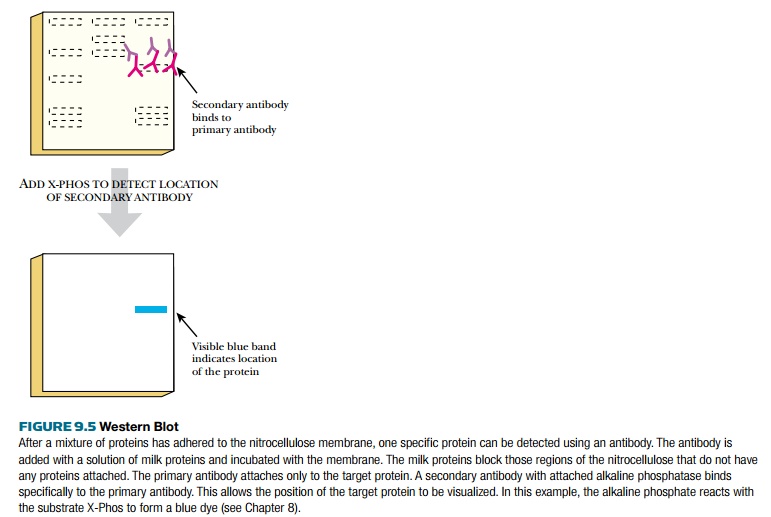
The next step is to visualize
the location of the primary antibody, thus revealing the location of the target
protein (Fig. 9.5). To achieve this, a secondary antibody is added. This
antibody recognizes the stem of the primary antibody without affecting its
binding to the protein. The secondary antibody has a tag or label on its stem
that is easily detected. Often the tag is the enzyme alkaline phosphatase,
which removes phosphate from various substrates. If the antibody complex is
incubated with a chromogenic substrate such as X-Phos, the alkaline phosphatase
removes the phosphate from the X-Phos. The remaining indolyl group reacts with
oxygen to form a blue precipitate in the location of the target protein. If a
chemiluminescent substrate is used, the nitrocellulose membrane is placed next
to a piece of photographic film. The light pulses turn the film black where the
secondary antibody/primary antibody/target protein complex is located on the
membrane. Western blots are used extensively both to prove that a protein is
being expressed and to estimate its level, because the intensity of the spot
directly correlates with the amount of protein.
Related Topics