Chapter: Biotechnology Applying the Genetic Revolution: Proteomics
High Pressure Liquid Chromatography Separates Protein Mixtures
HIGH-PRESSURE
LIQUID CHROMATOGRAPHY SEPARATES PROTEIN MIXTURES
Chromatography is a general term for many separation techniques, where a sample of molecules, the analyte, is dissolved in a mobile phase and then forced through a stationary phase. For 2D-PAGE, the mobile phase is the buffer and the stationary phase is the gels. In high-pressure liquid chromatography (HPLC) the sample is dissolved in a mobile phase and separated based on one specific characteristic by passing over a stationary phase (Fig. 9.6). In HPLC, the mobile phase is forced through a chromatography column, that is, a narrow tube packed with the stationary phase, under high pressure. As the mobile phase travels through the column, the mixture separates and different fractions are collected at the column exit. The mixture is forced through the column by constantly adding more mobile phase, in a process called elution. As the mobile phase exits the column, a detector emits a response to molecules in the eluting sample and draws a peak on the chromatogram.
HPLC has many applications
including separation, identification, purification, and quantification of
proteins or other analytes. Preparative HPLC is used to isolate and purify one
specific protein from a mixture. Using HPLC to identify a compound requires a
specific detection method. For example, if the target protein carries a
fluorescent label, then a fluorescence detector would be used. One application
of HPLC, quantitative HPLC, can be used to determine the amount of target
protein by comparing it to a set of standard proteins with known amounts. This
allows measurement of changes in level of a specific protein under different
conditions. A major benefit that HPLC offers to proteomics researchers is that
the separated proteins are already in a liquid state, making further analysis
easier.
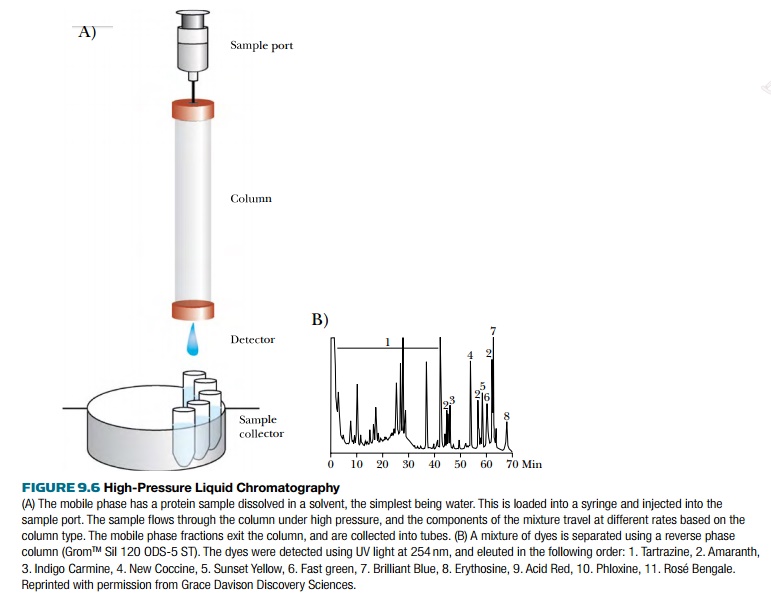
Although HPLC seems simple in
theory, the actual process of separating the mixture into its components is
complex. Each mixture has different chemistries, and so many different solid
phases are used to separate them. Even before it is loaded into the column, the
mixture can be manipulated to remove certain components or change their
chemistry. For example, treating with a phosphatase will remove phosphate
groups from proteins. Such manipulations can increase the efficiency with which
the protein of interest is isolated from the mixture.
HPLC is very adaptable
because of the availability of different types of stationary phase materials. Size exclusion chromatography columns
contain porous beads that separate mixtures of proteins by size. Large
molecules do not enter the pores of the beads and travel through the column
quickly, while smaller compounds are delayed by entering the beads. Many
different pore sizes are available for mixtures of different size ranges. Reverse phase HPLC uses columns packed with hydrophobic alkyl chains attached to
silica-based material. The column
binds and delays hydrophobic molecules while hydrophilic molecules elute
faster.
Ion-exchange HPLC uses a stationary phase with charged functional
groups that bind oppositely charged
molecules in the sample. Such molecules remain in the column after the sample
has passed through. To elute them, the mobile phase is changed. For example, if
the pH of the mobile phase is adjusted, the net charge on many proteins will be
altered and they will be released from the column. Other stationary phases form
hydrogen bonds with the analyte and separate based on overall polarity. For affinity HPLC, the stationary phase
contains a molecule that specifically binds the target protein, for example, an
antibody. When a mixture passes over the stationary phase, only the target
protein is bound, and other proteins pass through. Changing the mobile phase so
as to disrupt the interaction releases the protein of interest.
As molecules exit the column,
they must be detected. Many different detectors exist. These usually respond by
plotting peaks as molecules pass by. Refractive
index detectors monitor whether the exiting mobile phase refracts any light
by shining a light beam through it. Compounds present in exiting fractions will
scatter light, and a photodetector records this as a positive signal. The
amount of scatter affects the height of the peak and the length of time of the
scatter determines the width of the peak. Ultraviolet
detectors have a UV light source and a detector to determine when the
passing mobile phase absorbs the UV light. Such detectors may monitor one or
more wavelengths depending on the substance being examined. Fluorescence detectors detect compounds
that fluoresce, that is, absorb and re-emit light at different wavelengths; radiochemical detectors detect
radioactively labeled compounds; and electrochemical
detectors measure compounds that undergo oxidation or reduction reactions.
An approach that is increasingly used for proteomics is detection by mass
spectrometry. This combination allows proteins separated by HPLC to be fed
directly into a mass spectrometer for identification.
A critical aspect of HPLC is getting a good separation between the different proteins in the sample, that is, good resolution. Each peak that comes off the column should be symmetrical and as narrow as possible. For high resolution, many experimental conditions can be adjusted. The most obvious is changing the stationary phase. Sometimes just changing the particle size of the stationary phase improves separation. An alternative is to adjust the composition of the mobile phase. Temperature also affects many separations and may need to be controlled.
Related Topics