Chapter: Microbiology and Immunology: Antigen-Antibody Reactions
Western Blotting - Antigen Antibody Reactions
Western Blotting
Western blotting is called so because the procedure is similar to
Southern blotting, which was developed by Edwin Southern for the detection of
DNA. While Southern blotting is done to detect DNA, Western blotting is done
for the detection of proteins.
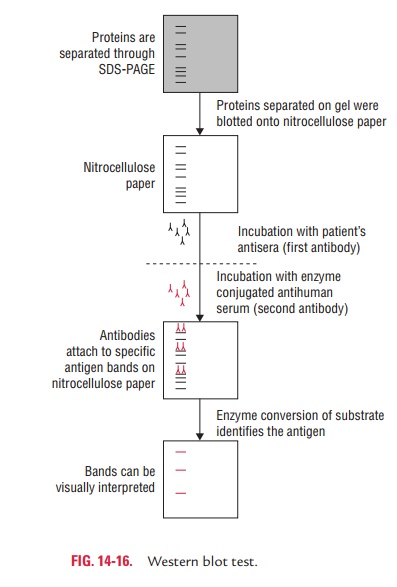
Western blotting is
usually done on a tissue homogenate or extract. It uses SDS-PAGE (sodium
dodecyl sulphate-polyacrylamide gel electrophoresis), a type of gel
electro-phoresis to first separate various proteins in a mixture on the basis
of their shape and size. The protein bands thus obtained are transferred onto a
nitrocellulose or nylon membrane where they are “probed” with antibodies
specific to the protein to be detected. The antigen–antibody com-plexes that
form on the band containing the protein recog-nized by the antibody can be
visualized in a variety of ways. If the protein of interest was bound by a
radioactive anti-body, its position on the blot can be determined by exposing
the membrane to a sheet of X-ray film, a procedure called autoradiography. However,
the most generally used detec-tion procedures employ enzyme-linked antibodies
against the protein. After binding of the enzyme–antibody conju-gate, addition
of a chromogenic substrate that produces a highly colored and insoluble product
causes the appear-ance of a colored band at the site of the target antigen. The
site of the protein of interest can be determined with much higher sensitivity
if a chemiluminescent compound along with suitable enhancing agents is used to
produce light at the antigen site (Fig. 14-16).
Related Topics