Chapter: Microbiology and Immunology: Antigen-Antibody Reactions
Types of agglutination reactions: Direct, Passive - Antigen Antibody Reactions
Types of agglutination reactions
Agglutination reactions where the antigens are found naturally on a
particle are known as direct agglutination. This is different from passive
agglutination, which employs particles that are coated with antigens not normally
found on their surfaces.
Direct agglutination
Direct
agglutination reactions can broadly be of the following types: (a) slide agglutination, (b) tube agglutination, (c) heterophile agglutination, and (d) antiglobulin (Coombs’) test.
Slide agglutination test: It is a basic type of agglutinationreaction
that is performed on a slide. Identification of bacterial types represents a
classic example of a direct slide agglutination that is still used today. In
this test, a suspension of bacteria is prepared and is added to a drop of
standardized antiserum. A positive reaction is indicated by clumping of
bacteria and clearing of the background solution. Clumping occurs instantly or
within seconds in a positive test. A control consisting of antigen suspension
in saline without adding antiserum is included on the same slide. It is used to
validate the results and also to detect possible false positives due to
autoagglutination of the antigen.
Tube agglutination test: Tube agglutination test, as
the namesuggests, is performed in glass tubes. Typically, in these tests,
patient’s serum is diluted in a series of tubes and bacterial antigens specific
for the suspected disease are added to it. Antigen and antibody reactions are
demonstrated by demonstration of visible clumps of agglutination. It is a
standard method used for quantitative estimation of antibodies in the serum.
Tube agglutination tests are routinely used for demonstration of antibodies in
the serum for serodiagnosis of enteric fever and brucellosis, as follows:
Heterophile agglutination test: This test depends on
demons-tration of heterophilic antibodies in serum present in certain bacterial
infections:
Antiglobulin (Coombs’) test: Coombs’ test was
devisedoriginally by Coombs’, Mourant, and Race for detection of incomplete
anti-Rh antibodies that do not agglutinate Rh1 erythrocytes in saline. When serum containing
incomplete anti-Rh antibodies is mixed with Rh1 erythrocytes in saline, incomplete antibody
antiglobulin coats the surface of erythrocytes but does not cause any
agglutination. When such erythrocytes are treated with antiglobulin or Coombs’
serum
·
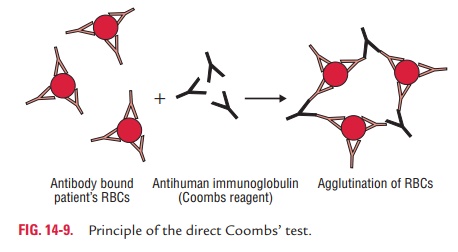
Direct Coombs’ test: In this test, the
sensitization of redblood cells (RBCs) with incomplete antibodies takes place invivo. The cell-bound antibodies can be
detected by this testin which antiserum against human immunoglobulin is used to
agglutinate patient’s red cells (Fig. 14-9).
·
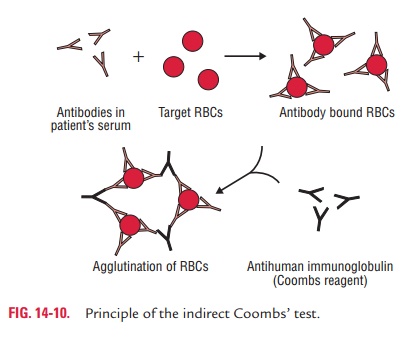
Indirect Coombs’ test: In this test, the
sensitization ofRBCs with incomplete antibodies takes place in vitro. In this test, the patient’s
serum is mixed with normal red cells and antiserum to human immunoglobulin is
added. Agglutina-tion occurs if antibodies are present in the patient’s serum
(Fig. 14-10).
Coombs’ tests are used for detection of (a) anti-Rh antibodies and (b)
incomplete antibodies in brucellosis and other diseases.
Passive agglutination
Passive agglutination employs carrier particles that are coated
with soluble antigens. This is usually done to convert precipitation reactions
into agglutination reactions, since the latter are easier to perform and
interpret and are more sensitive than precipitation reactions for detection of
anti-bodies. When the antibody instead of antigens is adsorbed on the carrier
particle for detection of antigens, it is called reversepassive agglutination.
Until the 1970s, erythrocytes were the major
carrier particles used for coating of antigens. Recently, however, a variety of
other particles including polystyrene latex, bentonite, and charcoal are used
for this purpose. Particle size vary from 7 m for RBCs to 0.05 m for
very fine latex particles. The use of synthetic beads or particles provides the
advantage of consistency, uniformity, and stability. Reactions are also easy to
read visually. Passive agglutination reac-tion, depending on the carrier
particles used, can be of the follow-ing types: (i) latex agglutination test, (ii)
hemagglutination test, and (iii)
coagglutination test.
Latex agglutination test: It is a test that employs
latex particlesas carrier of antigen or antibodies. In 1955, Singer and Plotz
accidentally found that IgG was naturally adsorbed to the surface of polystyrene
latex particles.
Latex particles are inexpensive, relatively stable and are not
subject to cross-reactivity with other antibodies. These particles can be
coated with antibodies to detect antigen in the serum and other body fluids.
Use of monoclonal antibodies has reduced the cross-reactions resulting in
reduction of false positive reactions.
Additionally, the large particle size of the latex facilitates
better visualization of antigen–antibody reactions by the naked eye
observation. The tests are usually performed on cardboard cards or glass slides
and positive reactions are graded on a scale of 11 to 41.
Hemagglutination test: RBCs are used as carrier
particlesin hemagglutination tests. RBCs of sheep, human, chick, etc. are
commonly used in the test. When RBCs are coated with antigen
to detect antibodies
in the serum,the
test is called indirect
hemagglutination (IHA) test.
The IHA is a most commonly used
test for serodiagnosis of many parasitic diseases including amoebiasis, hydatid
disease, and toxoplasmosis.
When antibodies are
attached to the RBCs to detect micro-bial antigen, it is known as reverse
passive hemagglutination(RPHA). The RPHA has been used extensively in
the pastto detect viral antigens, such as in HBsAg in the serum for diagnosis
of hepatitis B infection. The test has also been used for detection of antigens
in many other viral and parasitic infections.
Viral hemagglutination: Many viruses including influenza,mumps, and
measles have the ability to agglutinate RBCs with-out antigen–antibody
reactions. This process is called viral hemagglutination. This hemagglutination
can be inhibited by antibody specifically directed against the virus, and this
phenomenon is called hemagglutination inhibition. This
forms the basis of the viral hemagglutination inhibition test, which is used to
detect antibodies in patient’s sera that neutralize the agglutinating viruses.
To perform this test, patient’s serum is first incubated with a viral
preparation. Then RBCs that the virus is known to agglutinate are added to the
mixture. If anti-body is present, this will combine with viral particles and
prevent
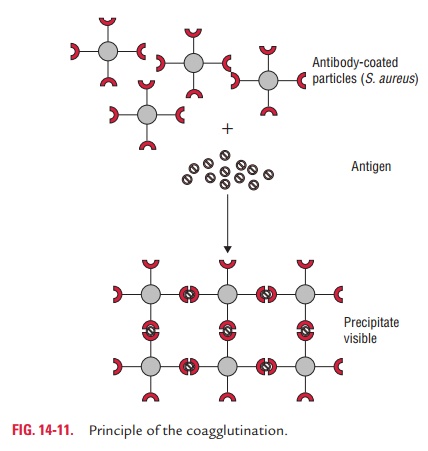
Coagglutination test: Coagglutination is a type
ofagglutination reaction in which Cowan I strain of S. aureus is used as carrier particle to coat antibodies. Cowan I
strain of S. aureus contains protein
A, an anti-antibody, that combineswith the Fc portion of immunoglobulin, IgG,
leaving the Fab region free to react with the antigen present in the specimens
(Fig. 14-11). In a positive test, protein A bearing S. aureus coated with antibodies will be agglutinated if mixed with
specific antigen. The advantage of the test is that these particles show
greater stability than latex particles and are more refractory to changes in
ionic strength.
Related Topics