Chapter: Aquaculture Principles and Practices: Health and Diseases
Viral diseases of aquaculture species
Viral diseases
Infectious pancreatic necrosis (IPN)
Infectious pancreatic necrosis is primarily a viral infection of trout and salmon, but the virus has also been isolated from a number of other fish species, including eels. IPN-like viruses have been isolated from common carp and several species of bivalve molluscs from coastal
waters. It is a major fish disease problem in North America and Europe and has been found also in Japan. It occurs as an acute disease in fry and fingerlings of trout. Culture technologies and management practices can affect the severity of disease outbreaks. When the mortality rate is high, infected individuals swim in a rotating manner about their long axis. This whirling behaviour is a terminal sign and death occurs within an hour or two. Prior to this stage, the affected individuals may remain on the bottom, showing weak respiration and convulsive movements. It has been observed that very young fish or fish in poor condition may not exhibit this characteristic whirling behaviour. An overall pale pigmentation of individual fish, exophthalmia, abdominal distension and haemorrhages in the ventral areas can be noticed (fig. 9.2). Haemorrhages also occur in the pyloric caecae, and the liver and spleen are usually pale. The occurrence of clear or milky mucus in the stomach and anterior intestine is a distinctive characteristic of IPN. Necrosis and inclusion bodies are evident histologically in the pancreatic tissue. Confirmation of the diagnosis requires isolation of the virus in cell
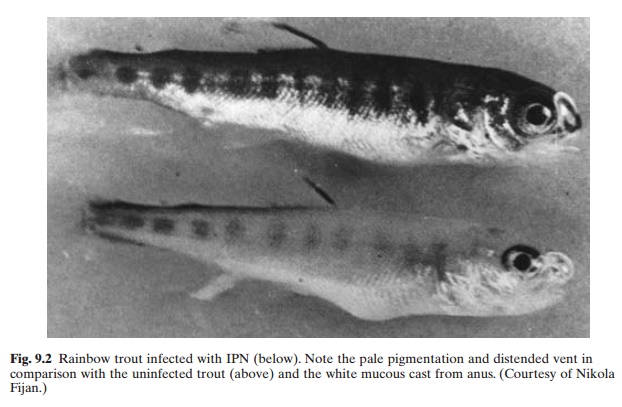
culture and identification by a serum neutralization test, using polyvalent anti-IPN virus serum.
IPN virus belongs to the birnavirus group. It grows in monolayers of fish cell cultures and induces a typical cytopathic effect. The incubation period is dependent on temperature, ranging from six days at 12.5°C to several weeks at 4°C. Most survivors of the infection become life-long virus carriers, intermittently shedding varying quantities of virus over a long period through urine, faeces, milt and eggs. This leads to the transmission of the virus from parents to progeny through the egg and accounts for transmission of the disease from one generation to another.
As there is no proven effective treatment for IPN, the only means of control is through preventive measures, which include the incubation of virus-free eggs and the propagation of IPN-free stock in uncontaminated water supplies. Rigorous fish health inspection programmes are essential to prevent inadvertent introduction of the disease.
Infectious haematopoietic necrosis (IHN)
Infectious haematopoietic necrosis is an acute viral disease of trout and salmon fry in North America and Japan. It is caused by a bullet-shaped virus and can be transmitted from fish to fish and from parent to progeny through seminal fluids or infected eggs. The disease is generally seen in fry and fingerlings, but this depends also on the host species. For example, in chinook salmon and steelhead and rainbow trout, mortality may occur from the sac fry stage to the yearling stage. Except for rainbow trout in certain areas, older fish rarely die from IHN.
Dark coloration, weakness, abdominal swelling and pale gills are some of the external signs of the disease. The internal signs are very similar to those caused by other viral infections. In infected sockeye salmon fry, the kidney becomes translucent and speckled with pigment cells. Diagnostic confirmation requires isolation and identification of the virus by neutralization tests with anti-IHNV serum IHN virus can reliably be detected only during the spawning season in carrier fish. Sometimes the IHN infection may be combined with that of IPN and so checks should be made for the presence of other viruses.
The primary mode of transmission is through infected eggs, but other means of transmission such as raw feedstuffs have also been recorded. Sockeye and Chinook salmon and rainbow and steelhead trout appear to be the most susceptible hosts. Coho salmon and other trout species are more resistant. Differing responses have been observed and are ascribed to interactions between strains of the virus, the amount of virus present and the species, strain and age of the host. The incubation period of IHN is temperature-dependent and ranges from 5.5 days at 21°C to about 16 days at 3°C.
No drugs or chemicals are known that will control IHN outbreaks. As in other virus diseases, prevention is the only means of control. The introduction of infected eggs and fish should be avoided. As carrier status for IHN can be reliably detected only at the spawning time and during epizootics, repeated inspections employing thorough virological samplings are necessary
Viral haemorrhagic septicaemia (VHS)
Viral haemorrhagic septicaemia is an acute to chronic viral disease of cultured salmonids, especially hatchery-reared rainbow trout, in Europe. The disease was first recognized in Germany in 1938 and later in Denmark, where it was called Egtved disease. The new name VHS was recommended in 1966 to reduce confusion. The disease is caused by a bullet-shaped rhabdovirus, very similar in size and shape to the IHN virus. The disease causes high mortality among rainbow trout fingerlings. If exposed for the first time, older fish are subject to chronic infection. Transmission is by contact and from fish to fish through water. As the water temperature rises, losses become less and cease during spring, recurring in autumn. Stress evoked by transportation or handling of trout can cause outbursts of the disease, with high mortality.
Early clinical signs of VHS can easily be confused with those of other viral, bacterial or parasitic infections. Acutely infected rainbow trout are dark in colour, lethargic and exhibit haemorrhages in the fin sockets. Exophthalmia is common and persists throughout the courseof the disease. With advance of the disease, the fish becomes nearly black and develops acute anaemia. The gills become pale in colour and bleeding occurs in gills and muscles (fig. 9.3). Signs of excitability can be seen, including erratic swimming similar to that of trout suffering from whirling disease caused by the protozoan parasite Myxosoma cerebralis. The diagnosis can be confirmed only by isolation and serological identification of the virus in an appropriate cell culture system. As in the case of IHN, the virus can be isolated from the carrier fish only during or soon after spawning. It can seldom be isolated from asymptomatic fish at other times.
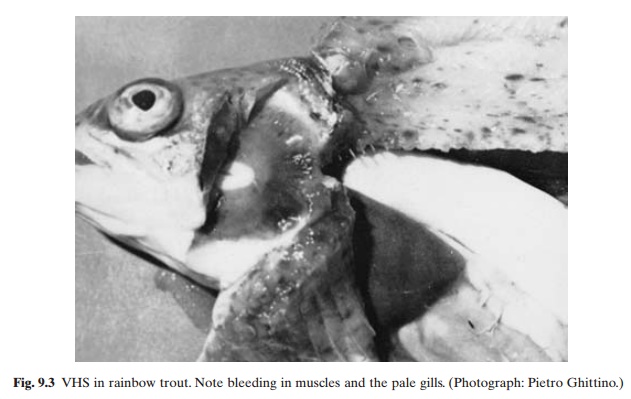
Although rainbow trout is the main species affected by VHS, other species also can be infected. Younger fish are more susceptible to the disease and most severe losses occur at the fingerling stage. Yearling fish generally suffer milder attacks and fish over two years old are almost completely resistant to infection. The disease is more serious at temperatures below 15–16°C. The incubation period appears to be about six days at 15°C and 8–11 days at about 10°C.
As there is no known cure for VHS, prevention is the best approach to control as in the case of other viral diseases. As there is strong circumstantial evidence that survivors of the epizootic become asymptomatic carriers, the transfer of such fish and the shipment of eggs contaminated by virusladen ovarian fluids should be avoided. Healthy fish should be isolated from possible sources of infection. If contaminated water has of necessity to be used in the farm, it should be thoroughly disinfected before use. The virus survives in water for more than 24 hours at 14°C.
Infectious dropsy of carp (IDC) and spring viremia of carp (SVC)
Infectious dropsy of carp was described by Schäperclaus in 1929 as the most serious disease of common carp and several other fish. The aetiology of IDC was controversial for many years. During the past 15 years it became evident that IDC includes several aetiologically different diseases, namely spring viremia of carp, carp erythrodermatitis, swim bladder inflammation, aeromonas septicaemia and

pseudomonas septicaemia. Methods of prevention and treatment of these diseases differ. Most researchers and diagnosticians have therefore discontinued use of the term IDC.
Spring viremia of carp (SVC) is an acute contagious disease caused by a rhabdovirus (fig. 9.4). The disease has so far only been recorded in Europe. It can cause high mortality in carp when the water temperature rises from 10 to
20°C in spring. Occasionally, SVC can also occur in late autumn or in winter. Mortalities cease at temperatures above 20–22°C. Silver carp and grass carp are also susceptible to SVC.
Signs of the disease include reduced movements and frequent resting, darkening of skin, bleeding in fins, skin, eyes and gills, faecal casts trailing from the protruding reddish anus, accumulation of fluid in the abdomen, protruding eyes, anaemia, inflamed intestine and bleeding in the internal organs. Diagnosis is confirmed by the isolation of the virus in cell cultures and its identification by a serum neutralization test. Secondary bacterial infections can aggravate the course of SVC and augment the losses.
The transmission of virus from brood fish to offspring can take place through eggs or sperm. The virus is relatively stable in water and mud. It enters the fish through the gills. Infection of the fish at water temperatures above 20°C results in an asymptomatic carrier state. Such carriers may have antibodies in the blood and are resistant to subsequent infection. Infected farms may therefore have few or no losses from SVC. Experiments indicate the possibility of SVC prevention by vaccination, but more research is needed before a safe and effective vaccine can be developed.
Eradication of SVC from large farms with a surface water supply is not possible. Small farms fed with well, spring or bore-hole water can be disinfected and kept free from SVC by the propagation of SVC-free stock.
Channel catfish virus disease (CCVD)
The most serious virus disease observed in channel catfish in culture facilities in the USA is caused by a herpes virus and occurs in fry and fingerlings less than four months old. It has been shown to be infective also in blue catfish (Ictalurus punctatus). The virus can be transmitted to fry and fingerlings through water in culture facilities. It is generally believed that channel catfish brood stock are carriers of the disease and they transmit the disease throughreproductive cells and/or fluids associated with reproduction. The virus has, however, not so far been detected in alleged carrier adults.
The disease occurs about 24 hours after infection, when water temperature ranges between 25 and 30°C. Affected fish may swim erratically or hang vertically in the water column with the head uppermost. The lesions begin at the posterior part of the kidney with an increasing number of lymphoid cells and proximal renal tubular necrosis. Focal necrotic lesions also develop in the liver, spleen and the digestive tract.The spleen is generally very dark and enlarged. Oedema and necrosis of the digestive tract result in massive sloughing of the intestinal lumen. Distension of the abdomen, exopthalmia and anaemia may also occur (fig. 9.5). Haemorrhages can be found in the muscles, gills, skin and fin bases. The disease is frequently associated with a secondary bacterial infection of Aeromonas hydrophila or Flex-ibacter columnaris. The virus retains infectivityin pond water for about two days at 25°C. The incubation period is about 32–72 hours at 30°C.
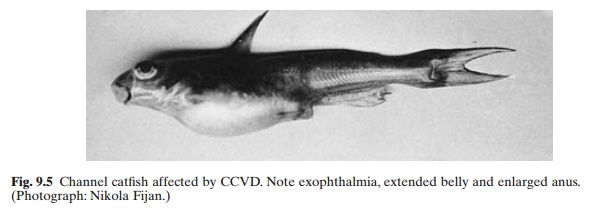
Since many of the symptoms of the disease are similar to other viral and bacterial diseases, the diagnosis has to be confirmed by isolation and identification of the virus. There is no known cure for the disease and no means of immunization has been developed, even though it has been observed that some individuals which survive the disease acquire a high level of immunity.The best control measures are pro-phylactic, including segregation from infected stocks and the use of uncontaminated water supplies. When disease occurs, infected stock should be removed and destroyed and the facilities thoroughly disinfected with a suitable disinfectant such as chlorine.
Carp pox (CP)
Carp pox is a relatively benign proliferative disease of cyprinids, known for more than 400 years in common carp in Europe. It is caused by a virus similar to a herpes virus. The disease is characterized by skin proliferation, which appears histologically as a plaque-warty hyper-plasia of the epidermis. In the advanced stages of the disease, mineral metabolism may be impaired and this can result in softness of the bones. Carp pox seldom causes mortality, but the cutaneous growth reduces marketability of the fish. Certain strains and inbred lines have a genetic predisposition to this disease. The carp louse (Argulus) can act as virus-carrier and transmit the virus within a pond population. The occurrence of carp pox can be reduced or eliminated by avoiding inbreeding or by genetic selection methods. No chemotherapy exists for the disease, but recovery can be speeded up by liming the ponds. It has been reported that infected carp recover if they are transferred to ponds supplied with large volumes of clear, oxygenated water (Ghittino, 1972).
Lymphocystis
Lymphocystis is a viral disease that occurs in several species of fresh-water, brackish-water and marine species of fish. It occurs as whitish nodules on the fins (fig. 9.6), head and, sometimes, the body of the fish. These are formed by the enlargement and encapsulation of the connective tissue cells. The disease is highly contagious and under culture conditions can spread very rapidly. It seldom causes any mortality, except when the verrucose lesions interfere with the ingestion of food. But the infected fish are difficult to sell, because of their appearance.
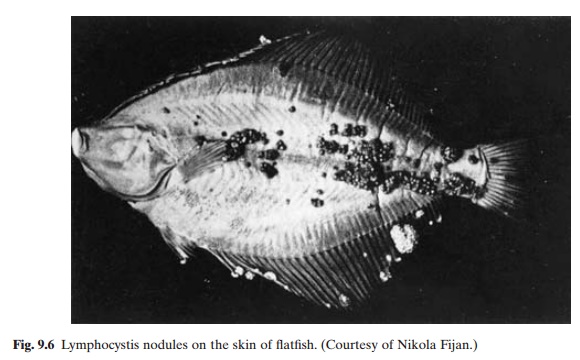
Like other viral diseases, lymphocystis also has no known cure and prophylaxis is the only means of control. Affected fish should be destroyed to prevent the spread of the virus to others, and the rearing facilities should be thoroughly disinfected.
Related Topics