Chapter: Aquaculture Principles and Practices: Health and Diseases
Protozoan diseases of aquaculture species
Protozoan diseases
Numerous protozoan parasites live on fish and other aquaculture species and cause both external and internal diseases, with serious mortalities in hatcheries and rearing facilities. Even moderate numbers of these organisms on small fish may prove fatal since the infections may cause the fish to stop feeding. Except in certain cases, the infected fish may die without showing any disease symptoms other than debilitation.
Ichthyophthiriasis (Ich)
Ichthyophthiriasis, caused by the protozoan parasite Ichthyophthirius multifiliis (fig. 9.16), is considered to be one of the most detrimental diseases in pond culture of fresh- and brackish-water fish. All species of pond-cultured fish
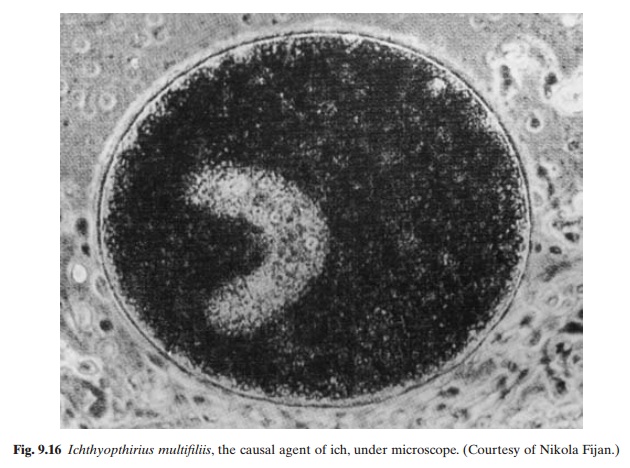
including common carp, Chinese carp and trout, are susceptible to the disease. I. multifiliis has a round or ovoid body (0.5–1.0 mm long)with a small rounded mouth. Longitudinal rows of cilia can be found on the surface of the body, converging at the anterior end. The large macronucleus is horseshoe-shaped and has numerous contractile vacuoles. This species multiplies on fish by repeated binary fission. The mature parasites (trophonts) break the white epithelial tubercle that covers them and enter the water. They settle at the bottom and attach themselves to submerged objects. On attachment, the parasite becomes enclosed in a gelatinous cyst and multiplies. One trophont divides into as many as 2000 ciliated bodies. They emerge into the water by dissolving the cyst which encloses them, with the enzyme hyaluronidase. They swim free for two to three days and if a host is found during that period, they penetrate under the skin, grow and mature. If they do not find a host, they will die. The optimal temperature for development is 25°–26°C. Outbreaks of the disease usually occur in spring and summer at high water temperature and under conditions of over-crowding that facilitate the spread of the disease. In light infections, the fish show rest-lessness and gather in groups near the water inlet. In heavy infections in yearlings, more severe symptoms can be noticed, including acute restlessness, the fish rubbing against the bottom and sides and collecting in masses near the inlet. Small tubercles occur on the body and the fish stop feeding and cease reacting to stimuli. In advanced cases, the fish swim at the surface and rush around swallowing air. Small white tubercles cover the entire body, and severe lesions of the cornea and blindness may also occur.
The fact that the non-parasitic stages of Ichthyophthirius are very sensitive to environmental factors makes it somewhat easier to prevent infection.The destruction of carrier fish is an essential aspect of prevention. Disinfection of contaminated water or equipment is recommended. Even dilute solutions of salt (0.5 per cent) will kill encysted parasites and the ciliated bodies. The ciliated bodies can also be killed by drying the ponds or other rearing facilities. A pH below 5 and oxygen concentrations of less than 0.8 mg/l are reported to be
fatal. Even though vaccines are not available for immunization of fish against ich, repeated infections apparently provide relative immunity. In ponds, treatment with 0.1 ppm malachite green or 15 ppm formalin once, twice or three times, has been reported to be effective.
Ichtyobodosis
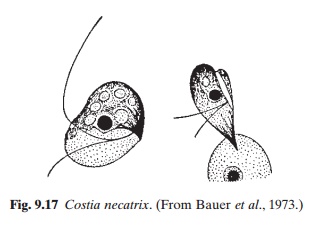
Ichtyobodosis, previously known as costiasis, is a severe disease affecting many species of fish, including common carp and trout, especially the younger age groups. It is caused by the flagellate Ichtyobodo necator (Costia necatrix) (fig. 9.17). The parasite attaches itself to the skin of the host. Its anterior end forms finger-shaped processes at the point of attachment, which penetrate into the cell of the host and suck its contents. The parasite multiplies by longitudinal division and dies when it falls from the host. Transmission takes place through water. Under adverse conditions, the body of the parasite becomes rounded.
It can live at temperatures ranging from 2 to 30°C or higher, but multiplies rapidly at 20–25°C. A pH of 4.5–5.5 is very favourable for mass reproduction. In carp farms, ichtyobodosis occurs frequently in spawning ponds at higher temperatures. The incidence of the disease decreases rapidly when the young fish are transferred to rearing ponds.
A characteristic symptom of the disease is the appearance of dull spots on the sides, which eventually become fused into a continuous grayish film by the increased secretion of mucus. The fins are frequently affected, starting with erosion of the tissue between the rays, which becomes exposed. The fry becomes emaciated and therefore the head looks enlarged. The infected gills are pale and covered with mucus. The fry rise to the surface and congregate near the inlet, swallowing air.
To avoid the incidence of the disease, young fish should be given nutritionally adequate food and should not be kept too long in spawning and rearing ponds. Carp spawning ponds should be disinfected with quicklime before spawners are introduced. Carrier fish should be eliminated and the water supply should be kept free from parasites. Ichtyobodo infections can be effectively treated in ponds with 15–50 ppm formalin, 2–3 ppm potassium permanganate or 0.1 ppm malachite green. A combination of 0.1 ppm malachite green with 15 ppm formalin has also been found to be effective.
Whirling disease
Whirling disease caused by the protozoan Myxosoma cerebralis is one of the well-known diseases of salmonids, and has been reported from many parts of the world including the whole of Europe, North and South America,Africa,Asia and New Zealand. It affects all species of trout and salmon, particularly the young. A common sign of the disease is rapid, tail-chasing behaviour when the fish are frightened or trying to feed. This is caused by the parasite feeding on the cartilage of young host fish. In advanced stages of the disease, skeletal deformation, including deformed heads, jaws and gill covers, as well as spinal curvature, can be observed. When exposed to the disease early in life, trout may develop ‘blacktail’. Acutely infected fry reared in contaminated water may not show any symptoms before high mortality sets in. When exposed, older fish exhibit less whirling behaviour. Again, fish with light infections may not show any signs at all, but will actually be carrying spores throughout their life. Confirmation of diagnosis has to be by isolation of spores or immature forms of the parasite in histological sections.
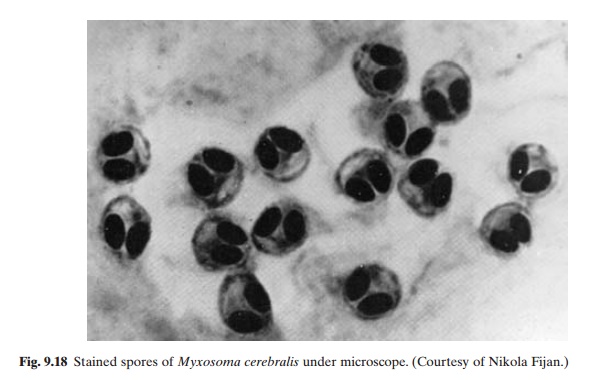
Infected fish, contaminated water and mud are known to be the reservoirs of infection. It has been reported that the spores may survive for 10–15 years in contaminated mud (Christensen, 1972). The exact route of infection has not yet been fully determined, but it would appear that the spores released by dead or living fish (fig. 9.18) develop infectivity in mud after a period of four to five months (Hoffman and Putz, 1969). It has been suggested that tubificid worms may be involved in the
While rainbow trout and Atlantic and kokanee salmon may become severely diseased by the parasite, brook trout, coho salmon and lake trout seem relatively resistant to the disease. The first 12 months of the life of the fish are the most susceptible period for infection. Fish between four and five months old do not develop acute clinical signs, even when infected, and may serve as asymptomatic carriers. Spore formation in infected fish takes about 52 days at 17°C. It may take about 28 weeks after exposure for symptoms of the disease to appear.
The only means of preventing infection by M. cerebralis is to prevent contact of susceptible fish with the parasite. The importation of infected fish or use of contaminated water should be avoided. Since the disease has become established in certain areas, it is extremely difficult to eradicate the pathogen. The use of resistant strains has been suggested as a management alternative. Through regulation of imports of fish and fish eggs by approved certification procedures, spread of the disease to uncontaminated areas can be controlled. There is no known effective therapy for the disease.
Other protozoan diseases
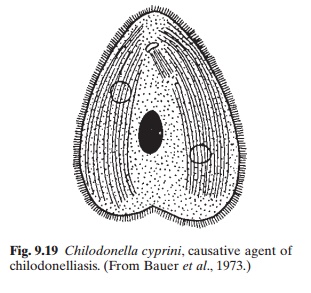
Of the other protozoan diseases, mention may be made of chilodonelliasis and trichodiniasis. Chilodonelliasis, caused by the ciliate Chilodonella cyprini (fig. 9.19), affects manyspecies of fish including young common carp and trout, causing heavy mortality in ponds. The parasite feeds on epithelial cells, which it pierces with its protrusible pharynx. It multiplies by transverse binary division, at a rapid rate, at an optimum temperature of 5–10°C. Temperatures above 20°C are lethal to the organism. Under adverse conditions, some individuals produce resting cysts. The cysts remain viable for a long time on the bottom or in the water until they find a host.
Emaciated or undernourished fish appear to be more susceptible to infection. The physiological condition of the fish and the abundance of the parasite are obviously important factors in epizootics of the disease. Transmission seems
In severe cases of infection, the body is covered with a bluishgrey film, distinctly noticeable on the dorsal side of the head. Heavily infested fish appear restless, rise to the surface, lose weight and become very lethargic. Yearling fish jump out of the water, due to impairment of cutaneous respiration.
Since the infection often takes place in wintering ponds, measures should be taken to prevent the parasites from entering these facilities. It is recommended that all fish should be given a five-minute bath of 5 per cent sodium chloride before transfer to wintering ponds.
Drying and disinfection with quicklime (2.5–4 tons/ha) would help to eliminate any cysts remaining on the bottom of the ponds from the fish wintered during the earlier season.
For curative treatment of infected fish, application of sodium chloride at a concentration of only 0.15–0.2 per cent for one to two days has been suggested. Since young salmonids may not tolerate high concentrations of sodium chloride, a bath of 0.005 per cent potassium permanganate for 10–15 minutes is recommended.
Trichodiniasis caused by Trichodina domerguei, T. pediculus, T. nigra, T. reticulata, T. epizootica and T. bulbosa evoke mortality in anumber of species of fish, including common carp and Chinese carps (grass carp, silver carp and bighead carp). The disease affects fry, fingerlings and yearlings of all pond fish. Adults are carriers of the disease.
The disease is transmitted either through contact with infected fish or via water. The body of the infected fish becomes dull, with a thin, whitish film of mucus, the quantity of which depends on the intensity of infection. In mild cases, the film is thin and restricted to the head and dorsum, but in severe cases the mucus covers the entire body and may flake off. At higher intensities of infection, the fish may become restless and congregate near the inlets.
As the infection progresses, mortality increases rapidly. Very often trichodiniasis, chilodonelliasis and ichthyophthiriasis occur together.
Preventive and curative treatments for the disease are similar to those for chilodonelliasis.
Related Topics