Chapter: Biochemistry: Biochemical Techniques
Types of chromatography
Types of chromatography
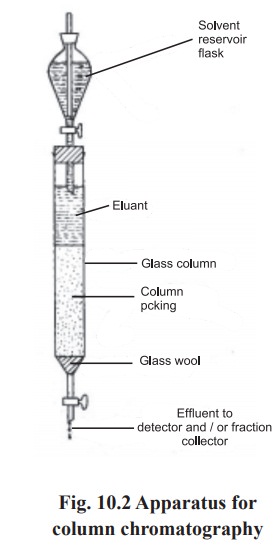
Column
chromatography: In this
type, the stationary phase is packed
into a glass or metal columns (wide tubes or cylinders). The mixture to be
separated is layered on the top of the column in the form of a solution at
particular concentration. After equilibration the components are eluted out of
the column one by one using specific mobile phases (Fig.10.2).The solvent used
to elute the separated components is known as eluant.
Thin
layer chromatography: In this
type, the stationary phase is thinly
coated on to a glass, plastic or foil plates. The mixture to be separated is
applied on the stationary phase at one end and kept vertical in the petridish
containing the mobile phase. When the mobile phase reaches the other end of the
plate the plate is removed from the petridish and the compounds separated are
identified by using specific staining reagents
Paper chromatography: In this type, the stationary column chromatography phase is
supported by the cellulose fibres of a paper sheet. The mobile phase flows
through the stationary phase and effects separation.
Each of these three types of chromatography
have their specific advantages, applications and method of operation.
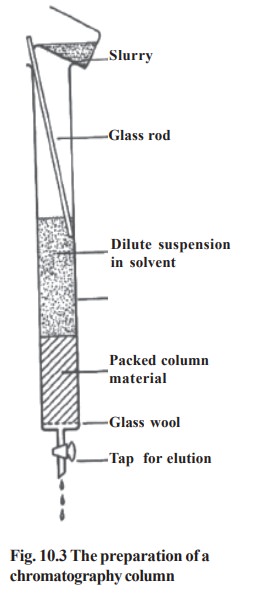
Column chromatography:
All the major types of chromatography are
routinely carried out using column
type (Fig. 10.3). The different types of column chromatography are
i. adsorption chromatography
ii. partition chromatography
iii. ion-exchange chromatography
iv. exclusion chromatography
v. affinity chromatography
i. Adsorption chromatography
Principle:
An adsorbent may be described as a solid which
has the property of adsorbing molecules at its surface, particularly when it is
porous and finely divided. Adsorption can be specific so that one solute may be
adsorbed selectively from a mixture. Separation of components by the method
depends upon differences both in their degree of adsorption by the adsorbent
and solubility in the solvent used for separation. Adsorption chromatography
can be carried out in both the column and thin layer modes.
ii.Partition chromatography Principle:
This technique is based on the partitioning of
compounds between a liquid stationary phase and a liquid mobile phase. The
liquid stationary phase can be held on any solid support like paper. This
technique is otherwise known as liquid- liquid chromatography.
iii. Gas liquid chromatography
Principle:
This technique is based upon the partitioning
of compounds between a liquid stationary
phase and a gas mobile phase . It is a widely used method for the qualitative
and quantitative analysis of a large number of compounds (eg. fatty acids)
because it has high sensitivity, reproducibility and speed of resolution. A stationary
phase of liquid material such as a silicone grease is supported on an inert
granular solid . This material is packed into a narrow coiled glass or steel
column 1 to 3 meter long and 2 to 4mm internal diameter. Through this column an
inert carrier gas (the mobile phase) such as nitrogen, helium or argon is
passed. The column is maintained in an oven at an elevated temperature which
volatilizes the compounds to be separated.
The basis for the separation is the difference
in the partition coefficients of the volatilized compounds between the liquid
and gas phases as the compounds are carried through the column by the carrier
gas. As the compounds flow, they leave the column and pass through a detector
which is connected to a recorder and record a peak. The area of the peak
corresponds to the concentration of the compound separated.
iv. Ion exchange chromatography
Principle:
The principle of this form of chromatography is
the attraction between oppositely charged
particles . Many biological materials, such as amino acids and proteins, have
ionisable groups and the fact that may carry a net positive or negative charge
can be utilized in separating mixtures of such compounds. The net charge
carried by such compounds depend on their pKa and on the pH of the solution.
Ion exchange separations are mainly carried out
in columns packed with an ion exchanger, which contain the core matrix molecule
with exchangeable ionic groups on its surface. There are two types of ion
exchangers, namely cation and anion exchangers. Cation exchangers posses
negatively charged groups and they will attract positively charged molecules.
Anion exchangers have positively charged groups which will attract negatively
charged molecules. The actual ion exchange mechanism composed of four steps;
·
selective
adsorption of the molecules to be separated by the ion exchange resins. B
release of the exchangeable group from the matrix.
·
Elution
of the absorbed molecule by specific eluants.
·
Regeneration
of the matrix by recharging with the original exchangeable groups.
Cation
exchanger
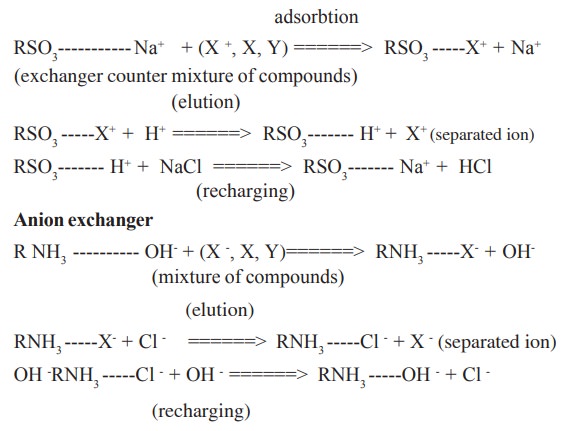
Some of the ion exchange materials used in this
technique are Amberlite IRC 50, Bio- Rex, Dowex 50, Sephadex etc.
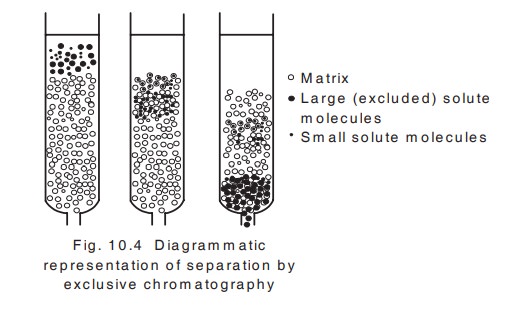
v. Molecular exclusion chromatography
This chromatography is otherwise known as gel
permeation chromatography.
Princple:
This technique is based on the separation of
molecules on the basis of their molecular
size and shape and the molecular sieve properties of a variety of porous
materials which serve as the solid stationary phase.
A column of gel particles or porous glass
granules is in equilibrium with a suitable solvent for the molecules to be
separated. Large molecules which are completely excluded from the pores will
pass through the interstitial spaces and smaller molecules will be distributed
between the solvent inside and outside the molecular sieve and will then pass
through the column at a lower rate . So the larger particles will come out of
the column first followed by smaller particles (fig. 10.4).
The gel materials generally used for this
technique are cross-linked dextrans, agarose, polyacrylamide, poly styrene etc.
vi. Affinity chromatography:
Principle:
This technique is based on the specific
biological interaction of the compounds to
be separated with the special molecules attached on the stationary phase called
as ligands. This technique requires that the material to be isolated is capable
of reversibly binding to a specific ligand which is attached to an insoluble
matrix (stationary phase).
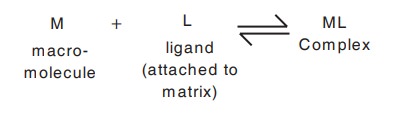
Under suitable experimental conditions when a
complex mixture containing the specific compound to be purified is added to the
insolubilised ligand generally contained in a chromatography column, only that
compound will bind to the ligand. All the other compounds can be washed away
and the compound subsequently recovered by displacement from the ligand. The
purification of an enzyme by this technique is shown diagrammatically in Fig.
10.5.
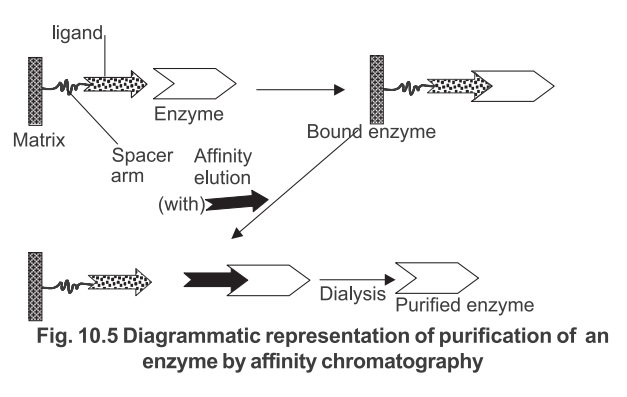
In practice, particles which are uniform,
spherical and rigid are used as matrix materials such as polystyrene,
cellulose, porous glass and silica etc.
Thin Layer chromatography
Principle:
Partition, adsorption, exclusion chromatography
can be carried out in a thin layer mode.
In this technique the stationary phase is made in the form of a slurry and
applied as a thin coating on the surface of a glass plate.After activating the
plate, the sample to be separated is applied at one end of the plate . The
plate is kept vertically in a chamber specially designed for this purpose( TLC
chamber) and allowed the sample and the mobile phase to raise through the
stationary phase by capillary action. The whole procedure consists of.
a. Thin
layer preparation : A slurry
of the stationary phase, generally applied to a glass, plastic or foil plate as a uniform thin layer by means of a plate
spreader starting from one end of the plate and moving progressively to the
other. Calcium phosphate is incorporated into the slurry in order to facilitate
the adhesion of the adsorbent to the plate. The plate is heated in an oven at
1000 C to activate the adsorbent.
b. Sample
application : The
sample is applied to the plate by means of a micropipette or syringe as spot or as a band on the
stationary phase.
c. Plate
development : Separation
takes place in a glass tank which contains mobile phase to a depth of about 1.5 cm. This is allowed to stand for atleast
an hour with a lid over the top of the tank to ensure that the atmosphere
within the tank becomes saturated with solvent vapour.
d. Component
detection : The
components separated are detected by (i) spraying the plate with 50% sulphuric acid or 25% sulphuric acid in ethanol and
heating; (ii) examining the plate under ultraviolet light; (iii) spraying of
plates with specific colour reagents, for example ninhydrin for amino acids.
Paper chromatography
Principle:
The cellulose fibres of chromatography paper
act as the supporting matrix for the stationary
phase. The stationary phase may be water or a non-polar material such as liquid
paraffin. The components get separated between the liquid stationary phase and
the liquid mobile phase. The procedure consists of
a. Paper
development: There
are two techniques which may be employed for the development of paper, ascending and descending methods. In both
cases, the solvent is placed in the base of a sealed tank or glass jar to allow
the chamber to become saturated with the solvent paper. The sample spots should
be in a position just above the surface of the solvent so that as the solvent
moves vertically up the paper by capillary action, separation of the sample is
achieved.
b. Component
detection: The
separated components can be detected by (i) examining the paper under ultraviolet light; (ii) spraying of papers with
specific colour reagents, for example ninhydrin for amino acids and sulphuric
acid for simple sugars.
The identification of a given compound may be
made on the basis of its Rf value (retardation factor) which is the
distance moved by the component during development divided by the distance
moved by the solvent from the point of origin (Fig. 10.6).
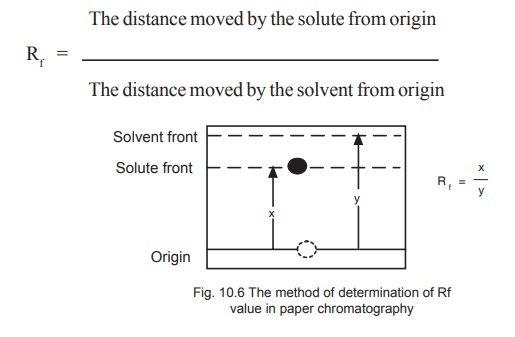
The value of Rf is constant for a
particular compound under standard conditions and closely reflects the
distribution co-efficient for that compound.
Related Topics