Chapter: Biochemistry: Biochemical Techniques
Paper chromatography
Paper chromatography
Principle: The cellulose fibres of chromatography paper act as the supporting matrix for the stationary phase. The stationary phase may be water or a non-polar material such as liquid paraffin. The components get separated between the liquid stationary phase and the liquid mobile phase. The procedure consists of
a. Paper development: There are two techniques which may be employed for the development of paper, ascending and descending methods. In both cases, the solvent is placed in the base of a sealed tank or glass jar to allow the chamber to become saturated with the solvent paper. The sample spots should be in a position just above the surface of the solvent so that as the solvent moves vertically up the paper by capillary action, separation of the sample is achieved.
b. Component detection: The separated components can be detected by (i) examining the paper under ultraviolet light; (ii) spraying of papers with specific colour reagents, for example ninhydrin for amino acids and sulphuric acid for simple sugars.
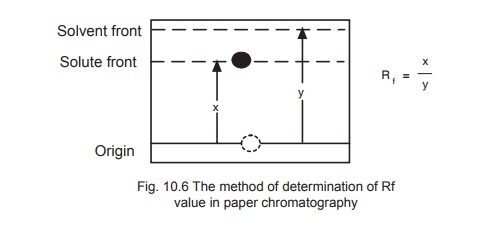
The identification of a given compound may be made on the basis of its Rf value (retardation factor) which is the distance moved by the component during development divided by the distance moved by the solvent from the point of origin (Fig. 10.6).
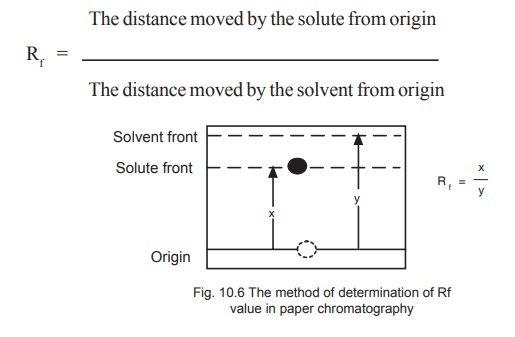
The value of Rf is constant for a particular compound under standard conditions and closely reflects the distribution co-efficient for that compound.
Related Topics