Chapter: Biochemistry: Biochemical Techniques
Spectrophotometry
Spectrophotometry
The present aim of the clinical chemists is the
development of micro and ultramicro –methodology for the analysis of all the
constituents of blood and body fluids. The study of functions of the body in
both health and diseases critically requires the quantitative analysis of blood
and body fluids for their various constituents.
Because so much of the quantitative methodology
of biological chemistry is based on colour or light measurement, consideration
must be given to the physical properties involved and to the fundamentals of
the instrumental procedures. Many methods for the quantitative analysis of
blood , tissue, urine, and other biological material are used on the separation
of the substance in question and its chemical conversion to a compound which is
capable of absorbing radiant energy. If the reaction product in solution
absorbs light in the visible region of the spectrum then the solution will be
coloured. The intensity or depth of colour of such a solution can be used as a
measure of concentration of the dissolved material. Determinations involving
quantitative estimation of colour are known as colorimetric analyses.
Many biochemical experiments involve the
measurement of the compound or group of compounds present in a complex.
Probably, the most widely used method for determining the concentration of
biochemical compounds is colorimetry which makes use of the property that when
white light passes through a coloured solution, some wave lengths are absorbed
more than others. Many compounds are not themselves coloured, but can be made
to absorb light in the visible region by reaction with suitable reagents.These
reactions are fairly specific and in most cases very sensitive, so that
quantities of materials in millimolar quantities can be measured.
A knowledge of the physical nature of colour
indicates that it is produced when specific regions or wavelengths of the
visible spectrum are absorbed. To take a simple example , a solution has blue
colour because , it absorbs a lesser proportion of the blue components of the
mixed white light passing through it than any other coloured components. Thus
the white light entering the solution will emerge in diminished intensity and
have a preponderance of the blue wave lengths so the solution appears to be
blue. The proportion of the various wave lengths of light absorbed is directly
related to the concentration of light absorbing material. The intensity of the
remaining transmitted colour is also a measure of the concentration of the
material present in the solution .
Analytical procedures based upon the direct
measurement of light absorption at specific wavelengths or regions of the
spectrum are known as photometric procedures and the instruments used are
photometers and spectrophotometers. In addition , there are methods which are
dependent on the ability of insoluble particles to scatter light, called
turbidometric methods and methods which are dependent on the ability of materials
to emit light under specified conditions, called fluorimetric methods.
Principle:
Spectrophotometric technique is based on the
basic laws of light absorption. For
uniform absorbing medium the proportion of the light radiation passing through
it is called the transmittance, T, where T=I/I0. I0 =
Intensity of the incident radiation, I= Intensity of the transmitted radiation.
The extent of radiation absorption is more commonly referred to as the
absorbance (A) or extinction (E) which are equal to the logarithm of the
reciprocal of the transmittance,
i.e., A = E = log 1/T = logI0/I
Transmittance is generally expressed on a range
of 0-100% and used in certain type of turbidity measurement. Absorbance or
extinction varies from 0 to ∞.
The Beer –Lambert Law
When a monochromatic light of initial intensity
I0 passes through a solution in a transparent vessel, some of the
light is absorbed so that the intensity of the transmitted light I is less than
I0 .There is some loss of light intensity from scattering by
particles in the solution and reflection at the interfaces, but mainly from
absorption by the solution. The relationship between I and Io
depends on the path length of the absorbing medium, l, and the concentration of the absorbing solution, c. These
factors are related in the laws of Lambert and Beer (Fig 10.9).
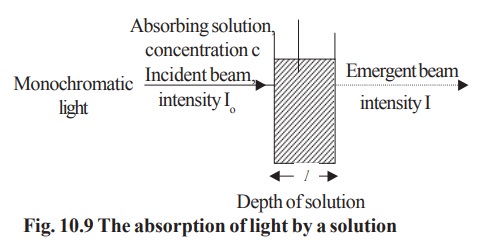
Lambert’s
law: When a ray of monochromatic
light passes through an absorbing medium
its intensity decreases exponentially as the length of the absorbing medium
increases.
I = I0 e- k1l
Beer’s
law : When a monochromatic light
passes through an absorbing medium its intensity decreases exponentially as the concentration of the absorbing
medium increases.
I = I0 e- k2 c
These two laws are combined together in the
Beer- Lambert law:
I = I0 e- k3
cl
Transmittance:
The ratio of intensities is known as the
transmittance (T) and this is usually expressed
as percentage
Percent T = I/I0 100 = e- k3cl
Extinction:
If logarithms are taken of the equation instead
of a ratio then
loge Io/ I = k3cl
log10 Io/I= k3cl / 2.303
log10 Io/I= kcl
The expression log10 Io/I is known
as the extinction (E) or absorbance(A). The extinction is some times referred
as optical density.
Therefore
A (or) E = k cl
where k is molar extinction co-efficient for
the absorbing material at wave length λ, c = molar concentration of the
absorbing solution, l = path length
in the absorbing material in cm. If the Beer- Lambert law is obeyed correctly
and l is kept constant, then a plot
of extinction against concentration gives a straight line passing through the
origin (Fig 10.10)
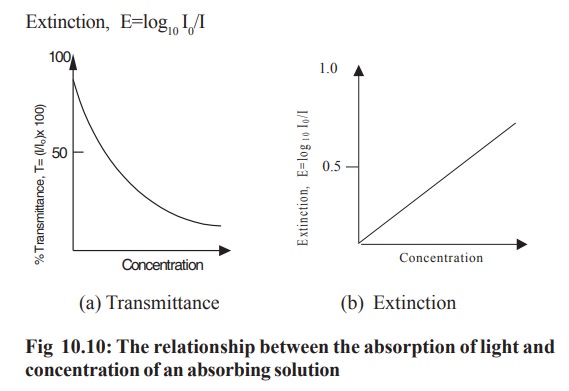
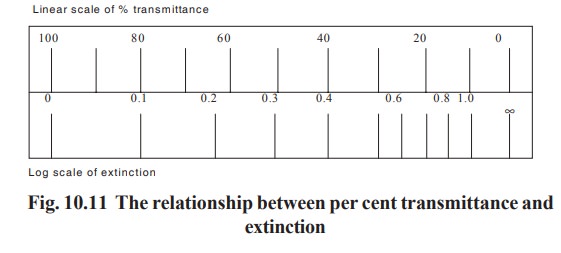
Some colorimeters and spectrophotometers have
two scales, a linear one of percent transmission and a logarithmic one of
extinction (Fig 10.11). The extinction scale is related linearly to the
concentration and this scale is used in the construction of a standard curve.
With the aid of such a curve the concentration of an unknown solution can
easily be determined from its molar extinction.
Molar extinction coefficient : If l is 1 cm and c is 1 mol/ litre then the
absorbance is equal to k, the molar extinction coefficient, which is
characteristics for a compound. The extinction coefficient k is thus the
extinction given by 1 mol / litre in a light path of 1 cm and usually written E1CM
, it has the dimention of mol-1 cm-1. The instruments
used for the measurement of extinction by the molecules to be quantified are
spectrophotometer and photoelectric colorimeters.
The photoelectric colorimeter:
A diagram of the basic arrangement of a typical colorimeter is given in Fig 10.12.
White light from a tungsten lamp passes through
a slit then a condenser lense, to give a parallel beam which falls on the
solution under investigation contained in absorption cell or cuvette. The cell
is made of glass with the sides facing the beam cut parallel to each other. In
most of the colorimeters, the cells are 1 cm square and will hold 5 ml of
solution .
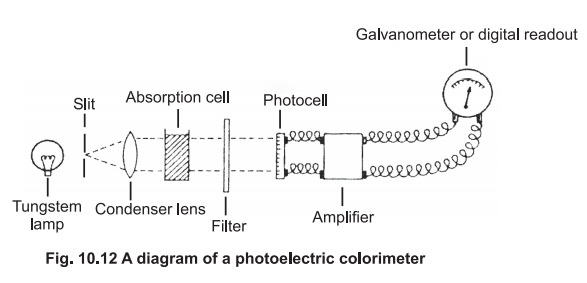
Beyond the absorption cell is the filter, which
is selected to allow maximum transmission of the colour absorbed. If a blue
solution is to be measured, a red filter should be selected.The colour of the
filter is, therefore, complementary to the colour of the solution under
investigation (Table 10.1). In some instruments the filter is located before
the absorption cell.
The light then falls on to a photocell which
generates an electrical current in direct proportion to the intensity of light
falling on it. This small electrical signal is increased in strength by the
amplifier , and the amplified signal passes to a galvanometer, or digital
readout, which is calibrated with logarithmic scale and the extinction can be
read directly. The blank solution (which does not contain the material under
investigation) is first taken in the cuvette and reading adjusted to zero
extinction and this is followed by the test solution and the extinction is
recorded directly.
A better method is to split the light beam ,
pass one part through the sample and the other through the blank, and balance
the two circuits to give zero. The extinction is determined from the
potentiometer reading which balances the circuit..
Photometric
analysis: There are four general steps
in carrying out a photometric analysis:
·
separation
of the substance from the complex mixture- for e.g., estimation of blood
glucose requires the precipitation of lipids and proteins by using
deproteinising agents which otherwise interfere with the colour reaction of
glucose
·
quantitative
conversion to a coloured or light absorbing substance-for e.g., after
deproteinisation as mentioned above for glucose estimation, the supernatant is
made to react with orthotoluidine reagent to give a greenish blue coloured
complex
·
measurement
of light absorption- for e.g., the colour intensity of the above mentioned
complex is measured by using a red filter.
·
calculation
of the concentration of the substance - for e.g., by comparing the extinction
with that of the standard solution of the same substance of known
concentration.

UV Absorption Spectrophotometry
A spectrophotometer is a sophisticated type of
colorimeter where monochromatic light is provided by a grating or prism in the
place of filter in ordinary colorimeter. The band width of the light passed by
a filter is quit broad, so that it may be difficult to distinguish between two
compounds of closely related absorption with a colorimeter. Some compounds
absorb strongly in the ultra violet region and their concentration can be
determined by using a more expensive type of spectrophotometer which operates
down to 190 nm. For e.g.,
·
The
activity of enzymes requiring NAD as coenzymes can be determined by treating
the enzyme source with the relevant substrate and measuring the NADH formed (colourless)
which gives strong absorption at 340 nm. The increase in absorbance is
proportional to the concentration of the enzyme.
·
the
concentration of uric acid can be estimated by measuring the extinction of the
solution at 293 nm before and after treatment with an excess of the enzyme
uricase. At pH 9.0, uric acid which absorbs at 293 nm, is oxidized by uricase
to allantoin, which has no absorption at this wave length. The decrease in
absorbance at 293 nm is a measure of uric acid level.
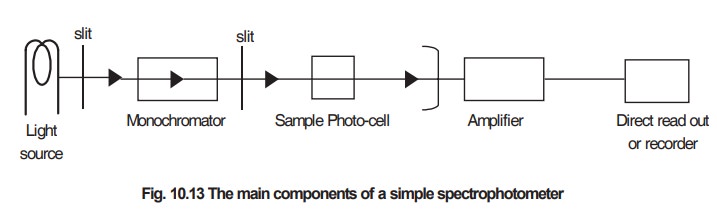
The main components of a simple spectrophotometer
are shown in Fig 10.13
Absorption spectra
Many compounds have characteristic absorption
spectra in the ultra violet and visible regions so that identification of those
materials in a mixture is possible.
Proteins:
Proteins absorb strongly at 280 nm according to
their content of the amino acids tyrosine
and tryptophan, and this provides a sensitive and non-destructive form of
assay.
Nucleic
acids: Nucleic acids and their
component bases show maximum absorption in the region of 260nm. The extent of absorption of nucleic acid is a
measure of their integrity, since the partial degraded acids absorb more
strongly than the native materials.
Haem
proteins: These conjugated proteins
absorb in the visible region as well as in the UV region of the spectrum due to haem group. The visible spectra of
the oxidized and reduced forms of cytochrome C are sufficiently different so
that the relative amounts of these forms can be determined in a mixture.
Things
to remember: The
detailed operation of a particular instrument must be obtained by carefully reading the instruction
manual. Few important points concerning the use and care of calorimeters and
spectrophotometers are given below.
·
Cleaning
the cuvette:The cuvette should be cleaned by soaking in 50 per cent v/v nitric
acid and then thoroughly rinsed in distilled water.
·
Using
the cuvette: First of all, fill the cuvette with distilled water and check them
against each other to correct for any small difference in optical properties.
Always wipe the outside of the cuvette with soft tissue paper before placing in
the cell holder. When all the measurement have been taken, wash them with
distilled water and leave in the inverted position to dry.
·
Absorption
of radiation by cuvettes: All cuvettes absorb radiation and the wave length at
which significant absorption occurs depend on the material from which the
cuvette is made. Silica cuvettes are the most transparent to U/V light but they
are expensive. Glass cuvettes are much cheaper than silica, and so they are used
whenever possible and invariably in the visible region of the spectrum.
·
Light
source : A tungsten lamp produces a broad range of radient energy down to about
360 nm. To obtain the ultra violet region of the spectrum a deuterium lamp is
used as the light source.
·
Blanks:
The extinction of a solution is read against a reagent blank which contains all
the reagents except the compound to be measured. The blank is first placed in
the instrument and the scale adjusted to zero extinction before reading any
solution . Alternatively, the extinction can be read against distilled water
and the blank reading can be subtracted from that of the test solution
·
Duplicates:
It is essential to prepare all blanks, standard solutions and unknown solutions
in duplicates so that the accurate standard curve can be obtained.
·
Construction
of standard curve : A series of concentrations of standard solution are taken
in different test tubes and made to react with colouring agents. The blank tube
is also treated similarly but by replacing standard solution with water. The
absorbance are measured at the corresponding wavelength and a graph is plotted
as concentration of the standard versus the absorbance.
Applications of spectrophotometry
Colorimetry and spectrophotometry have widest
application in biological sciences. These techniques are used for the
determination of
·
glucose,
proteins, lipids, nucleic acid etc
·
turbidity
of solutions( bacterial cell mass)
·
absorption
spectrum of a compound
·
purity
of compound by knowing the molar extinction coefficient which is maximum for a
pure compound.
Related Topics