Chapter: Biochemistry: Biochemical Techniques
The photoelectric colorimeter
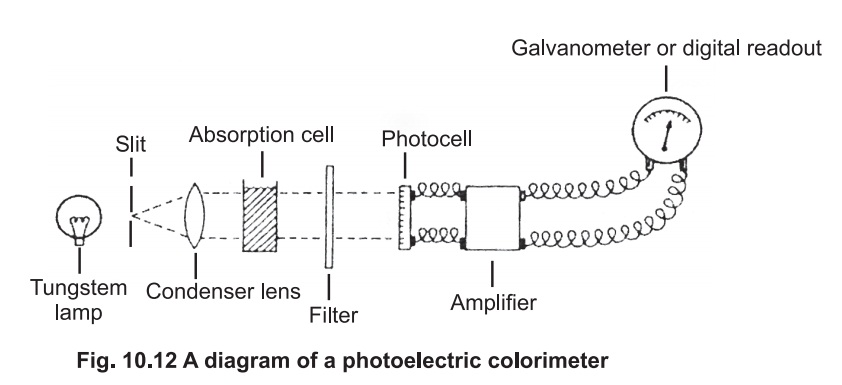
The photoelectric colorimeter:
A diagram of the basic arrangement of a typical colorimeter is given in Fig 10.12.
White light from a tungsten lamp passes through
a slit then a condenser lense, to give a parallel beam which falls on the
solution under investigation contained in absorption cell or cuvette. The cell
is made of glass with the sides facing the beam cut parallel to each other. In
most of the colorimeters, the cells are 1 cm square and will hold 5 ml of
solution .
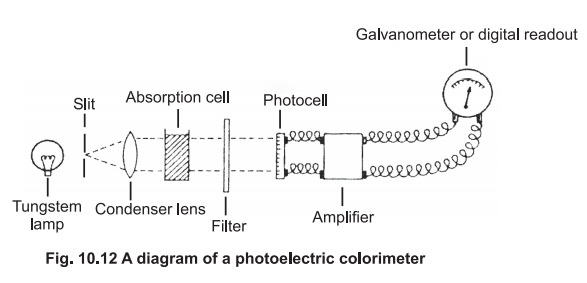
Beyond the absorption cell is the filter, which
is selected to allow maximum transmission of the colour absorbed. If a blue
solution is to be measured, a red filter should be selected.The colour of the
filter is, therefore, complementary to the colour of the solution under
investigation (Table 10.1). In some instruments the filter is located before
the absorption cell.
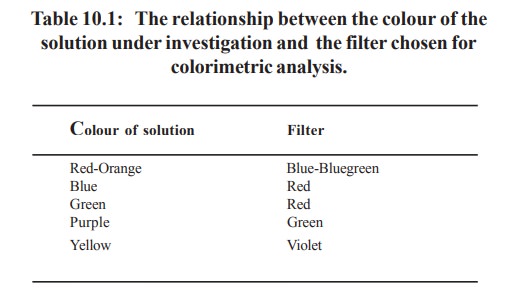
The light then falls on to a photocell which
generates an electrical current in direct proportion to the intensity of light
falling on it. This small electrical signal is increased in strength by the
amplifier , and the amplified signal passes to a galvanometer, or digital
readout, which is calibrated with logarithmic scale and the extinction can be
read directly. The blank solution (which does not contain the material under
investigation) is first taken in the cuvette and reading adjusted to zero
extinction and this is followed by the test solution and the extinction is
recorded directly.
A better method is to split the light beam ,
pass one part through the sample and the other through the blank, and balance
the two circuits to give zero. The extinction is determined from the
potentiometer reading which balances the circuit..
Photometric
analysis: There are four general steps
in carrying out a photometric analysis:
·
separation
of the substance from the complex mixture- for e.g., estimation of blood
glucose requires the precipitation of lipids and proteins by using
deproteinising agents which otherwise interfere with the colour reaction of
glucose
·
quantitative
conversion to a coloured or light absorbing substance-for e.g., after
deproteinisation as mentioned above for glucose estimation, the supernatant is
made to react with orthotoluidine reagent to give a greenish blue coloured
complex
·
measurement
of light absorption- for e.g., the colour intensity of the above mentioned
complex is measured by using a red filter.
·
calculation
of the concentration of the substance - for e.g., by comparing the extinction
with that of the standard solution of the same substance of known
concentration.

UV Absorption Spectrophotometry
A spectrophotometer is a sophisticated type of
colorimeter where monochromatic light is provided by a grating or prism in the
place of filter in ordinary colorimeter. The band width of the light passed by
a filter is quit broad, so that it may be difficult to distinguish between two
compounds of closely related absorption with a colorimeter. Some compounds
absorb strongly in the ultra violet region and their concentration can be
determined by using a more expensive type of spectrophotometer which operates
down to 190 nm. For e.g.,
·
The
activity of enzymes requiring NAD as coenzymes can be determined by treating
the enzyme source with the relevant substrate and measuring the NADH formed (colourless)
which gives strong absorption at 340 nm. The increase in absorbance is
proportional to the concentration of the enzyme.
·
the
concentration of uric acid can be estimated by measuring the extinction of the
solution at 293 nm before and after treatment with an excess of the enzyme
uricase. At pH 9.0, uric acid which absorbs at 293 nm, is oxidized by uricase
to allantoin, which has no absorption at this wave length. The decrease in
absorbance at 293 nm is a measure of uric acid level.
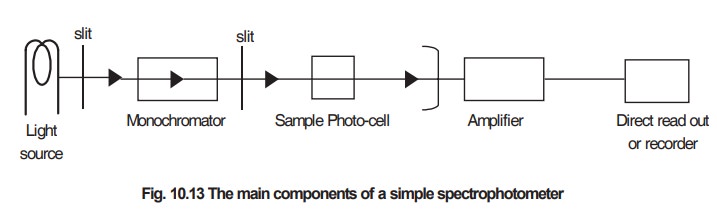
The main components of a simple spectrophotometer
are shown in Fig 10.13
Absorption spectra
Many compounds have characteristic absorption
spectra in the ultra violet and visible regions so that identification of those
materials in a mixture is possible.
Proteins:
Proteins absorb strongly at 280 nm according to
their content of the amino acids tyrosine
and tryptophan, and this provides a sensitive and non-destructive form of
assay.
Nucleic
acids: Nucleic acids and their
component bases show maximum absorption in the region of 260nm. The extent of absorption of nucleic acid is a
measure of their integrity, since the partial degraded acids absorb more
strongly than the native materials.
Haem
proteins: These conjugated proteins
absorb in the visible region as well as in the UV region of the spectrum due to haem group. The visible spectra of
the oxidized and reduced forms of cytochrome C are sufficiently different so
that the relative amounts of these forms can be determined in a mixture.
Things
to remember: The
detailed operation of a particular instrument must be obtained by carefully reading the instruction
manual. Few important points concerning the use and care of calorimeters and
spectrophotometers are given below.
·
Cleaning
the cuvette:The cuvette should be cleaned by soaking in 50 per cent v/v nitric
acid and then thoroughly rinsed in distilled water.
·
Using
the cuvette: First of all, fill the cuvette with distilled water and check them
against each other to correct for any small difference in optical properties.
Always wipe the outside of the cuvette with soft tissue paper before placing in
the cell holder. When all the measurement have been taken, wash them with
distilled water and leave in the inverted position to dry.
·
Absorption
of radiation by cuvettes: All cuvettes absorb radiation and the wave length at
which significant absorption occurs depend on the material from which the
cuvette is made. Silica cuvettes are the most transparent to U/V light but they
are expensive. Glass cuvettes are much cheaper than silica, and so they are used
whenever possible and invariably in the visible region of the spectrum.
·
Light
source : A tungsten lamp produces a broad range of radient energy down to about
360 nm. To obtain the ultra violet region of the spectrum a deuterium lamp is
used as the light source.
·
Blanks:
The extinction of a solution is read against a reagent blank which contains all
the reagents except the compound to be measured. The blank is first placed in
the instrument and the scale adjusted to zero extinction before reading any
solution . Alternatively, the extinction can be read against distilled water
and the blank reading can be subtracted from that of the test solution
·
Duplicates:
It is essential to prepare all blanks, standard solutions and unknown solutions
in duplicates so that the accurate standard curve can be obtained.
·
Construction
of standard curve : A series of concentrations of standard solution are taken
in different test tubes and made to react with colouring agents. The blank tube
is also treated similarly but by replacing standard solution with water. The
absorbance are measured at the corresponding wavelength and a graph is plotted
as concentration of the standard versus the absorbance.
Related Topics