Chapter: Biochemistry: Biochemical Techniques
Chromatography
Chromatography
One of the tasks of biochemists is to identify,
separate and purify one or more biological components in a mixture of such
compounds in a biological sample. One of the most important convenient methods
for achieving such separation is the use of chromatographic techniques.
The term chromatorgraphy was originally applied
by Micheal Tswett, a Russian Botanist, in 1906 to a procedure where a mixture
of different coloured pigments (chlorophylls and xanthophylls) was separated
from each other.
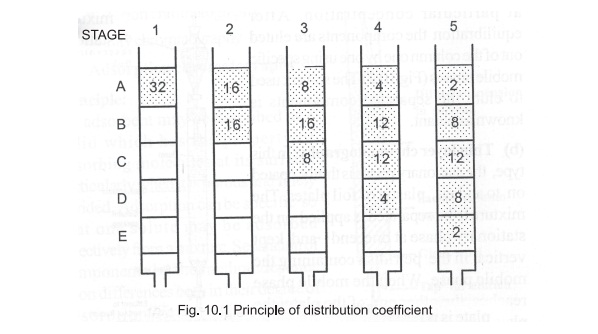
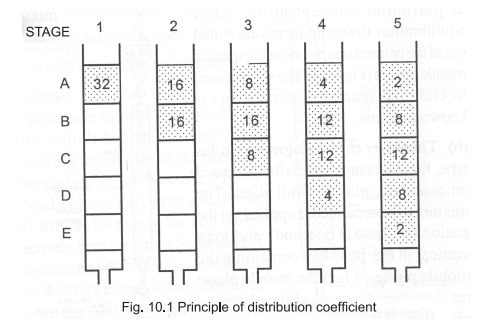
Principle
: The basis of all forms of chromatography is the
partition or distribution co-efficient
which describes the way in which a compound distributes itself between two
immiscible phases. For a compound distributing itself between equal volumes of
two immiscible solvents A and B (Fig. 10.1), the value of distribution
co-efficient is a constant at a given temperature and is given by the
expression
Basically all chromatographic systems consists
of two phases. One is the stationary phase which may be a solid, liquid or a
solid liquid mixture which is immobilized. The mobile phase may be a liquid or a
gas and flows over or through the stationary phase.
Separation starts to occur when a compound to
be separated is held more firmly by the stationary phase than the other which
tends to move on slower in the mobile phase. Thus, the underlying principle of
chromatorgraphy is to adsorb the components of the mixture on an insoluble
material and then to differentially remove or elute these components one by one
with suitable solvents.
The term effective distribution co-efficient is
defined as the total amount as distinct from the concentration of substance
present in one phase divided by the total amount present in the other phase.
Thus, a distribution co-efficient of a substance between alumina (stationary
phase) and butanol (mobile phase) might be 0.25 which means that the
concentration of the substance in butanol is four times that in the alumina.The
choice of stationary or mobile phases is made so that the compounds to be
separated have different distribution co-efficient.
In practice separations may be achieved by
using different types of chromatographic techniques
Related Topics