Chapter: Basic & Clinical Pharmacology : Pancreatic Hormones & Antidiabetic Drugs
Thiazolidinediones
THIAZOLIDINEDIONES
Thiazolidinediones (Tzds) act to decrease insulin resistance. Tzds are ligands of peroxisome proliferator-activated receptor-gamma (PPAR-f), part of the steroid and thyroid superfamily ofnuclear receptors. These PPAR receptors are found in muscle, fat, and liver. PPAR-γ receptors modulate the expression of the genes involved in lipid and glucose metabolism, insulin signal transduc-tion, and adipocyte and other tissue differentiation. The available Tzds do not have identical clinical effects, and new drug develop-ment will focus on defining PPAR effects and designing ligands that have selective action—much like the selective estrogen recep-tor modulators .
In addition to
targeting adipocytes, myocytes, and hepatocytes, Tzds also have significant
effects on vascular endothelium, the immune system, the ovaries, and tumor
cells. Some of these responses may be independent of the PPAR-γ pathway. The Tzd
oncogenic effects are complex and may be both tumorigenic and antitumorigenic.
In persons with
diabetes, a major site of Tzd action is adipose tissue, where the drug promotes
glucose uptake and utilization and modulates synthesis of lipid hormones or
cytokines and other proteins involved in energy regulation. Tzds also regulate
adipo-cyte apoptosis and differentiation. Numerous other effects have been
documented in animal studies, but applicability to human tissues has yet to be
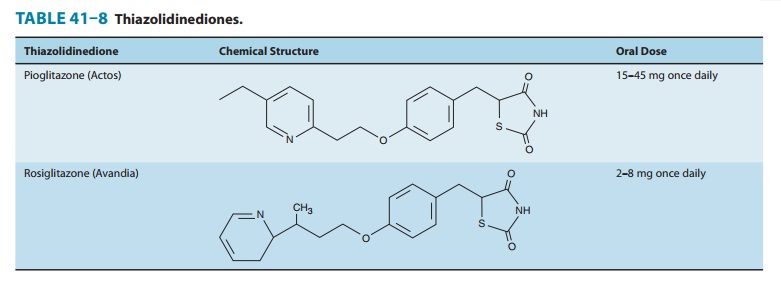
determined.Two thiazolidinediones are currently available:
pioglitazone and rosiglitazone (Table 41–8). Their distinct side chains create
differences in therapeutic action, metabolism, metabolite profile, and adverse
effects. An earlier compound, troglitazone, was with-drawn from the market
because of hepatic toxicity thought to be related to its side chain.
Pioglitazone has PPAR-αas well as PPAR-γactivity. It isabsorbed within 2 hours of
ingestion; although food may delay uptake, total bioavailability is not affected.
Absorption is decreased with concomitant use of bile acid sequestrants.
Pioglitazone is metabolized by CYP2C8 and CYP3A4 to active metabolites. The
bioavailability of numerous other drugs also degraded by these enzymes may be
affected by pioglitazone therapy, including estro-gen-containing oral
contraceptives; additional methods of contra-ception are advised. Pioglitazone
may be taken once daily; the usual starting dose is 15–30 mg/d, and the maximum
is 45 mg/d. A triglyceride-lowering effect is observed with pioglitazone and is
more significant than that of rosiglitazone, presumably because of its PPAR-α-binding
characteristics. Pioglitazone is approved as a monotherapy and in combination
with metformin, sulfonylureas, and insulin for the treatment of type 2
diabetes. The risk of blad-der cancer appears to be cumulatively increased with
high doses.
Rosiglitazone is rapidly absorbed and highly protein-bound.It is metabolized
in the liver to minimally active metabolites, pre-dominantly by CYP2C8 and to a
lesser extent by CYP2C9. It is administered once or twice daily; 2–8 mg is the
usual total dose.
Rosiglitazone shares
the common Tzd adverse effects but appears to carry more cardiovascular risk
than pioglitazone. Concurrent administration of nitrates and insulin putatively
enhances the risk of myocardial infarction and is contraindicated;
renin-angiotensin system blockers may have a similar risk but are not
specifically prohibited. Because of toxicities, the FDA now limits
rosiglitazone prescriptions to patients already on treatment with the
medica-tion, and to those whose blood sugar cannot be controlled with other
antidiabetic medicines and who, after consultation with their provider, do not
wish to be on pioglitazone or a pioglitazone-containing drug. For those restricted populations,
rosiglitazone isapproved for use in type 2 diabetes as monotherapy, in double
combi-nation therapy with a biguanide or sulfonylurea, or in quadruple
combination with a biguanide, sulfonylurea, and insulin.
Tzds are considered
euglycemics and are efficacious in about 70% of new users. The overall response
is similar to that achieved with sulfonylurea and biguanide monotherapy.
Individuals experi-encing secondary failure with other oral agents should
benefit from the addition (rather than substitution) of a Tzd. Because their
mechanism of action involves gene regulation, the Tzds have a slow onset and
offset of activity over weeks or even months. Combination therapy with
sulfonylureas and insulin can lead to hypoglycemia and may require dosage
adjustment.
An adverse effect
common to both Tzds is fluid retention, which presents as a mild anemia and
peripheral edema, especially when the drugs are used in combination with
insulin or insulin secretagogues. Both drugs increase the risk of heart
failure. Many users have a dose-related weight gain (average 1–3 kg), which may
be fluid related. Rarely, new or worsening macular edema has been reported in
association with treatment. Loss of bone mineral den-sity and increased
atypical extremity bone fractures in women are described for both compounds,
which is postulated to be due to decreased osteoblast formation. Studies are
ongoing to determine whether the demineralization and fracture risk is
increased in men. Long-term therapy is associated with a drop in triglyceride
levels and a slight rise in high-density lipoprotein (HDL) and low-density
lipoprotein (LDL) cholesterol values. These agents should not be used during
pregnancy or in the presence of significant liver disease (ALT more than 2.5
times upper limit of normal) or witha concurrent diagnosis of
heart failure. Because of the hepatotoxicity observed with troglitazone, a
discontinued Tzd, the FDA continues to require monitoring of liver function
tests before initiation of Tzd therapy and periodically afterward. To date,
hepatotoxicity has not been associated with rosiglitazone or pioglitazone.
Anovulatory women may resume ovulation and should be coun-seled on the
increased risk of pregnancy.
Thiazolidinediones
have benefit in the prevention of
type 2 dia-betes. The Diabetes Prevention Trial reported a 75% reduction in
diabetes incidence rate when troglitazone was administered to patients with
prediabetes. Another study reported that troglitazone therapy significantly
decreased the recurrence of diabetes mellitus in high-risk Hispanic women with
a history of gestational diabetes.
Although these
medications are highly efficacious, the adverse effects of weight gain,
congestive heart failure, demineralization and increased bone fracture in women;
possible (for rosiglitazone) worsening of cardiovascular status; and unknown
oncogenic risk potentially limit their popularity and future use.
Related Topics