Chapter: Basic & Clinical Pharmacology : Pancreatic Hormones & Antidiabetic Drugs
Glucagon
GLUCAGON
Chemistry & Metabolism
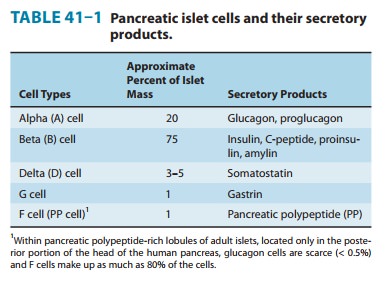
Glucagon is
synthesized in the alpha cells of the pancreatic islets of Langerhans (Table
41–1). Glucagon is a peptide—identical in all mammals—consisting of a single
chain of 29 amino acids, with a molecular weight of 3485. Selective proteolytic
cleavage converts a large precursor molecule of approximately 18,000 MW to
glu-cagon. One of the precursor intermediates consists of a 69-amino-acid
peptide called glicentin, which
contains the glucagon sequence interposed between peptide extensions.
Glucagon
is extensively degraded in the liver and kidney as well as in plasma and at its
tissue receptor sites. Because of its rapid inactivation by plasma, chilling of
the collecting tubes and addition of inhibitors of proteolytic enzymes are
necessary when samples of blood are collected for immunoassay of circulating
glucagon. Its half-life in plasma is between 3 and 6 minutes, which is similar
to that of insulin.
“Gut Glucagon”
Glicentin
immunoreactivity has been found in cells of the small intestine as well as in
pancreatic alpha cells and in effluents of perfused pancreas. The intestinal
cells secrete enteroglucagon, a
family of glucagon-like peptides, of which glicentin is a member, along with
glucagon-like peptides 1 and 2 (GLP-1 and GLP-2). Unlike the pancreatic alpha
cell, these intestinal cells lack the enzymes to convert glucagon precursors to
true glucagon by removing the carboxyl terminal extension from the molecule.
Glucagon-like Peptide 1 (GLP-1)
The function of the
enteroglucagons has not been clarified, although smaller peptides can bind
hepatic glucagon receptors where they exert partial activity. A derivative of
the 37-amino-acid form of GLP-1 that lacks the first six amino acids
(GLP-1[7–37]) is a potent stimulant of insulin synthesis and release and
beta-cell mass. In addition, it inhibits glucagon secretion, slows gastric
emptying, and has an anorectic effect. After oral glucose ingestion, GLP-1
along with another gut hormone, GIP, accounts for as much as 70% of the induced
insulin secretion. GLP-1 represents the predominant form of GLP in the human
intestine and has been termed insulinotropin.
It has been considered as a potential therapeutic agent in type 2 diabetes.
However, GLP-1 requires continuous subcutaneous infusion to produce a sustained
lower-ing of both fasting and postprandial hyperglycemia in type 2 dia-betic
patients; therefore, its clinical usefulness is limited. Exenatide and
liraglutide (see previous text) are GLP-1 receptor agonist ana-logs with more
practical half-lives.
Pharmacologic Effects of Glucagon
A. Metabolic Effects
The
first six amino acids at the amino terminal of the glucagon molecule bind to
specific Gs protein-coupled receptors on
livercells. This leads to an increase in cAMP, which facilitates catabo-lism of
stored glycogen and increases gluconeogenesis and keto-genesis. The immediate
pharmacologic result of glucagon infusion is to raise blood glucose at the
expense of stored hepatic glycogen. There is no effect on skeletal muscle
glycogen, presumably because of the lack of glucagon receptors on skeletal
muscle. Pharmacologic amounts of glucagon cause release of insulin from normal
pancre-atic beta cells, catecholamines from pheochromocytoma, and cal-citonin
from medullary carcinoma cells.
B. Cardiac Effects
Glucagon has a potent
inotropic and chronotropic effect on the heart, mediated by the cAMP mechanism
described above. Thus, it produces an effect very similar to that of β-adrenoceptor
ago-nists without requiring functioning β receptors.
C. Effects on Smooth Muscle
Large doses of
glucagon produce profound relaxation of the intes-tine. In contrast to the
above effects of the peptide, this action on the intestine may be due to
mechanisms other than adenylyl cyclase activation.
Clinical Uses
A. Severe Hypoglycemia
The
major use of glucagon is for emergency treatment of severe hypoglycemic
reactions in patients with type 1 diabetes when unconsciousness precludes oral
feedings and intravenous glucose treatment is not possible. Recombinant
glucagon is currently available in 1-mg vials for parenteral use (Glucagon
Emergency Kit). Nasal sprays have been developed for this purpose but have not
yet received FDA approval.
B. Endocrine Diagnosis
Several
tests use glucagon to diagnose endocrine disorders. In patients with type 1
diabetes mellitus, a classic research test of pancreatic beta-cell secretory
reserve uses 1 mg of glucagon administered as an intravenous bolus. Because
insulin-treated patients develop circulating anti-insulin antibodies that
interfere with radioimmunoassays of insulin, measurements of C-peptide are used
to indicate beta-cell secretion.
C. Beta-Adrenoceptor Blocker Overdose
Glucagon is sometimes
useful for reversing the cardiac effects of an overdose of β-blocking agents
because of its ability to increase cAMP production in the heart. However, it is
not clinically useful in the treatment of cardiac failure.
D. Radiology of the Bowel
Glucagon
has been used extensively in radiology as an aid to X-ray visualization of the
bowel because of its ability to relax the intestine.
Adverse Reactions
Transient nausea and
occasional vomiting can result from gluca-gon administration. These are
generally mild, and glucagon is relatively free of severe adverse reactions. It
should not be used in a patient with pheochromocytoma.
Related Topics