Chapter: Basic & Clinical Pharmacology : Pancreatic Hormones & Antidiabetic Drugs
Insulin
INSULIN
Chemistry
Insulin
is a small protein with a molecular weight in humans of 5808. It contains 51
amino acids arranged in two chains (A and B) linked by disulfide bridges; there
are species differences in the amino acids of both chains. Proinsulin, a long
single-chain protein molecule, is processed within the Golgi apparatus of beta
cells and packaged into granules, where it is hydrolyzed into insulin and a
residual connecting segment called C-peptide by removal of four amino acids
(Figure 41–1).
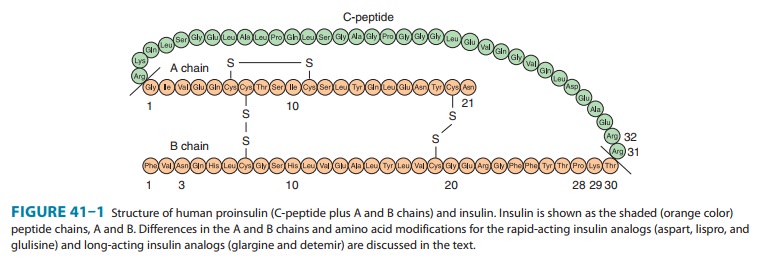
Insulin and C-peptide
are secreted in equimolar amounts in response to all insulin secretagogues; a
small quantity of unpro-cessed or partially hydrolyzed proinsulin is released
as well. Although proinsulin may have some mild hypoglycemic action, C-peptide
has no known physiologic function. Granules within the beta cells store the
insulin in the form of crystals consisting of two atoms of zinc and six
molecules of insulin. The entire human pancreas contains up to 8 mg of insulin,
representing approximately 200 biologic units. Originally, the unit was defined
on the basis of the hypogly-cemic activity of insulin in rabbits. With improved
purification techniques, the unit is presently defined on the basis of weight,
and present insulin standards used for assay purposes contain 28 units per
milligram.
Insulin Secretion
Insulin is released
from pancreatic beta cells at a low basal rate and at a much higher stimulated
rate in response to a variety of stimuli, especially glucose. Other stimulants
such as other sugars (eg, mannose), amino acids (especially gluconeogenic amino
acids, eg, leucine, arginine), hormones such as glucagon-like polypeptide-1
(GLP-1), glucose-dependent insulinotropic poly-peptide (GIP), glucagon,
cholecystokinin, high concentrations of fatty acids, and β-adrenergic
sympathetic activity are recognized. Stimulatory drugs are sulfonylureas,
meglitinide and nateglinide, isoproterenol, and acetylcholine. Inhibitory
signals are hormones including insulin itself and leptin, α-adrenergic
sympathetic activity, chronically elevated glucose, and low concentrations of
fatty acids. Inhibitory drugs include diazoxide, phenytoin, vin-blastine, and
colchicine.
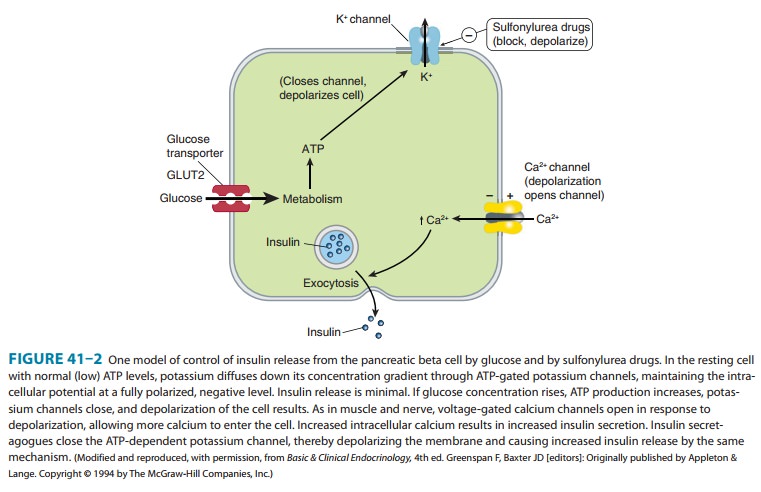
One
mechanism of stimulated insulin release is diagrammed in Figure 41–2. As shown
in the figure, hyperglycemia results in increased intracellular ATP levels,
which close the ATP-dependent potassium channels. Decreased outward potassium
efflux results in depolarization of the beta cell and opening of voltage-gated
calcium channels. The resulting increased intracellular calcium triggers
secretion of the hormone. The insulin secretagogue drug group (sulfonylureas,
meglitinides, and D-phenylalanine) exploits parts of
this mechanism.
Insulin Degradation
The liver and kidney are the two main organs that remove insulin from the circulation. The liver normally clears the blood of approximately 60% of the insulin released from the pancreas by virtue of its location as the terminal site of portal vein blood flow, with the kidney removing 35–40% of the endogenous hormone. However, in insulin-treated diabetics receiving subcutaneous insulin injections, this ratio is reversed, with as much as 60% of exogenous insulin being cleared by the kidney and the liver removing no more than 30–40%. The half-life of circulating insu-lin is 3–5 minutes.
Circulating Insulin
Basal insulin values
of 5–15 μU/mL
(30–90 pmol/L) are found in normal humans, with a peak rise to 60–90 μU/mL (360–540 pmol/L)
during meals.
The Insulin Receptor
After insulin has
entered the circulation, it diffuses into tissues, where it is bound by
specialized receptors that are found on the membranes of most tissues. The
biologic responses promoted by these insulin-receptor complexes have been
identified in the pri-mary target tissues, ie, liver, muscle, and adipose
tissue. The recep-tors bind insulin with high specificity and affinity in the
picomolar range. The full insulin receptor consists of two cova-lently linked
heterodimers, each containing an α subunit, which is entirely extracellular and
constitutes the recognition site, and a subunit that spans the membrane (Figure
41–3). The β
subunit contains a tyrosine kinase. The binding of an insulin molecule to the α subunits at the
outside surface of the cell activates the receptor and through a conformational
change brings the catalytic loops of the opposing cytoplasmic β subunits into closer
proximity. This facilitates mutual phosphorylation of tyrosine residues on the
subunits and tyrosine kinase activity directed at cytoplasmic proteins.
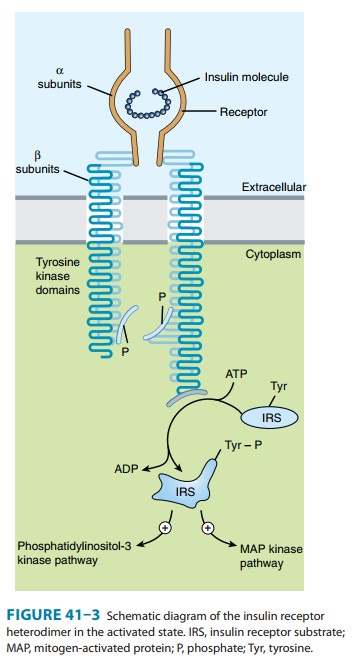
The first proteins to
be phosphorylated by the activated recep-tor tyrosine kinases are the docking
proteins, insulin receptor substrates (IRS). After tyrosine phosphorylation at
several critical sites, the IRS molecules bind to and activate other
kinases—most significantly phosphatidylinositol-3-kinase—which produce fur-ther
phosphorylations. Alternatively, they may bind to an adaptor protein such as
growth factor receptor-binding protein 2, which translates the insulin signal
to a guanine nucleotide-releasing fac-tor that ultimately activates the GTP
binding protein, ras, and the mitogen-activated protein kinase (MAPK) system.
The particular IRS-phosphorylated tyrosine kinases have binding specificity
with downstream molecules based on their surrounding 4–5 amino acid sequences
or motifs that recognize specific Src homology 2 (SH2) domains on the other
protein. This network of phospho-rylations within the cell represents insulin’s
second message and results in multiple effects, including translocation of
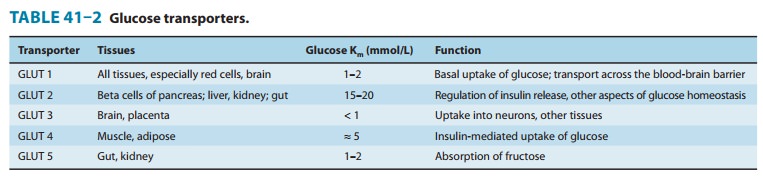
glucose trans-porters (especially GLUT 4, Table 41–2) to the cell membrane with
a resultant increase in glucose uptake; increased glycogen synthase activity
and increased glycogen formation; multiple effects on protein synthesis,
lipolysis, and lipogenesis; and activation of
Various hormonal agents (eg, glucocorticoids) lower the affinity of insulin receptors for insulin; growth hormone in excess increases this affinity slightly. Aberrant serine and threonine phosphorylation of the insulin receptor β subunits or IRS molecules may result in insulin resistance and functional receptor down-regulation.
Effects of Insulin on Its Targets
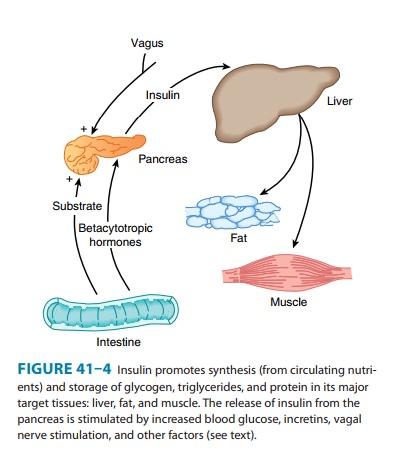
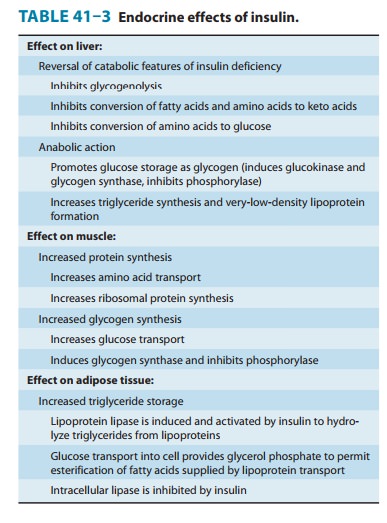
Insulin promotes the
storage of fat as well as glucose (both sources of energy) within specialized
target cells (Figure 41–4) and influ-ences cell growth and the metabolic
functions of a wide variety of tissues (Table 41–3).
Related Topics