Chapter: Genetics and Molecular Biology: Nucleic Acid and Chromosome Structure
The Regular Backbone of DNA
The Regular Backbone Of DNA
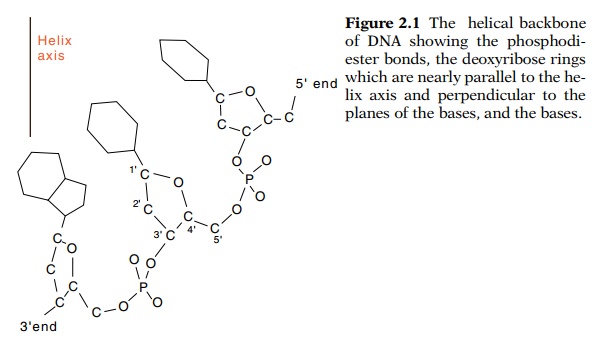
The chemical structure of DNA is a regular backbone
of 2’-deoxyriboses, joined by 3’-5’ phosphodiester bonds (Fig. 2.1). The
information carried by the molecule is specified by bases attached to the 1’
position of the deoxyriboses. Four bases are used: the purines adenine and
guanine, and the pyrimidines cytosine and thymine. The units of base plus
ribose or deoxyribose are called nucleosides, and if phosphates are attached to
the sugars, the units are called nucleotides.
The chemical structure of RNA is similar to that of
DNA. The backbone of RNA uses riboses rather than 2’-deoxyriboses, and the
methyl group on the thymine is absent, leaving the pyrimidine uracil.
Clearly the phosphate-sugar-phosphate-sugar along
the backbones of DNA and RNA are regular. Can anything be done to make the
informa-tion storage portion of the molecule regular as well? At first glance
this seems impossible because the purines and the pyrimidines are different
sizes and shapes. As Watson and Crick noticed however, pairs of these
molecules, adenine-thymine and guanine-cytosine, do possess regular shapes
(Fig. 2.2). The deoxyribose residues on both A-T and G-C pairs are separated by
the same distance and can be at the same relative orientations with respect to
the helix axis. Not only are these pairs regular, but they are stabilized by
strong hydrogen bonds. The A-T pair generally can form two hydrogen bonds and
the G-C base pair can form
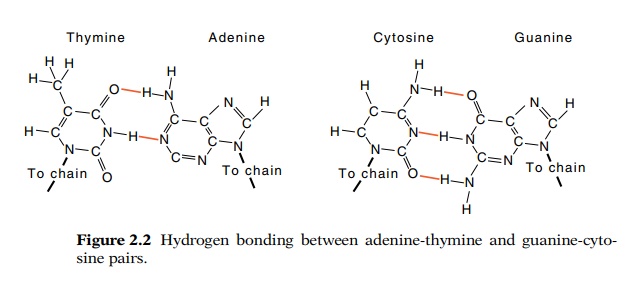
Hydrogen bonds can form when a hydrogen atom can be
shared by a donor such as an amino group and an acceptor such as a carbonyl
group. The hydrogen bonds between the bases of DNA are strong because in all
cases the three atoms participating in hydrogen bond formation lie in nearly
straight lines. In addition to the familiar Wat-son-Crick pairings of the
bases, other interactions between the bases have been observed and are also
biologically important. These alterna-tive structures frequently occur in tRNA
and also are likely to exist in the terminal structures of chromosomes, called
telomeres.
Related Topics