Chapter: Genetics and Molecular Biology: Nucleic Acid and Chromosome Structure
Measuring Superhelical Turns
Measuring Superhelical Turns
Superhelical turns in DNA may introduce distortions
or torsion in the molecules that assist or hinder processes we would like to
study such as recombination or the initiation of transcription. Supercoiling
must be easily measurable in order to be productively studied. One way to
measure superhelical turns might be to observe the DNA in an electron
microscope and see it twisted upon itself. Quantitation of the superheli
turns in DNA with more than a few turns is difficult however. More convenient measurement methods exist. Consider the DNA molecule just described with 100 negative superhelical turns. Because it is so twisted upon itself, the molecule is rather compact and sediments in the ultracentrifuge at a high rate. If the sedimentation is performed in the presence of a low concentration of ethidium bromide, a few molecules will intercalate into the DNA. This will reduce the number of negative superhelical turns, thereby opening up the DNA, which will sediment more slowly than it would in the absence of ethidium bromide.
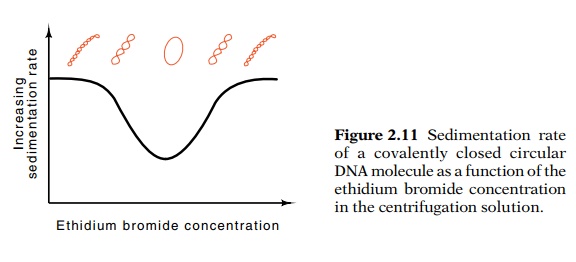
Figure 2.11 Sedimentation rateof a covalently closed circular DNA molecule as a function of the ethidium bromide concentration in the centrifugation solution.
Consider
a series of sedimentation measurements made in the pres-ence of increasing
concentrations of ethidium bromide. At higher and higher concentrations of
ethidium bromide, more and more will inter-calate into the DNA and unwind the
DNA more and more. Consequently the DNA will become less and less compact and
sediment more and more slowly (Fig. 2.11). Finally a concentration of ethidium
bromide will be reached where the molecule is completely free of superhelical
turns. At this concentration, the DNA will sediment most slowly. If the
centrifugation is done in the presence of still higher concentrations of
ethidium bromide, the molecule will be found to sediment more rapidly as the
DNA acquires positive superhelical turns and becomes more compact again. The
concentration of ethidium bromide required to generate the slowest
sedimentation rate can then be related to the number of superhelical turns
originally in the DNA via the affinity of ethidium bromide for DNA and the
untwisting produced per interca-lated ethidium bromide molecule.
Even more
convenient than centrifugation for quantitation of super-helical turns has been
electrophoresis of DNA through agarose. Under some conditions DNA molecules of
the same length but with different linking numbers can be made to separate from
one another upon electrophoresis. The separation results from the fact that two
molecules with different linking numbers will, on the average during the
electro-phoresis, possess different degrees of supercoiling and consequently
different compactness. Those molecules that are more greatly super-coiled
during the electrophoresis will migrate more rapidly. Not only can agarose gels
be used for quantitating species with different numbers of superhelical turns,
but any particular species can be extracted out of the gel and used in
subsequent experiments.
The
agarose gels show an interesting result. If DNA is ligated to form covalently
closed circles and then subjected to electrophoresis under conditions that
separate superhelical forms, it is found that not all of the DNA molecules
possess the same linking number. There is a distri-bution centered about the
linking number corresponding to zero super-helical turns, Lk0. This is to be expected because the DNA molecules in
solution are constantly in motion, and a molecule can be ligated into a
covalently closed circle at an instant when it possesses a linking number
unequal to Lk0. These
molecules are frozen in a slightly higher average energy state than those with
no superhelical turns. Their exact energy depends on the twisting spring
constant of DNA. The stiffer the DNA, the smaller the fraction of molecules
that will possess any superhelical turns at the time of sealing. Quantitation
of the DNA molecules in the bands possessing different numbers of superhelical
turns permits evalu-ation via statistical mechanics of the twisting spring
constant of DNA.
The ability to measure accurately the number of
superhelical turns in DNA allows a determination of the amount of winding or
unwinding produced by the binding of molecules. For example, unwinding meas -
urements first indicated that RNA polymerase melts about 8 bases of DNA when it
binds tightly to lambda DNA. Later, more precise meas-urements have shown that
the unwinding is closer to 15 base pairs. This unwinding was shown directly by
binding RNA polymerase to nicked circular DNA and then sealing with ligase to
form covalently closed circles, removing the RNA polymerase, and measuring the
number of superhelical turns in the DNA. The first measurements were done by
accurately comparing the sedimentation velocity of the DNA sealed in the
presence and in the absence of RNA polymerase. Later experiments have used a
better DNA substrate and have used gel electrophoresis.
Another way to measure the winding produced by
binding of a molecule to DNA is to measure the affinity of a molecule for DNA
samples containing different numbers of superhelical turns. This method is
based on the fact that a protein which introduces negative superhelical turns
as it binds to DNA will bind much more tightly to a DNA molecule already
containing negative superhelical turns. From the thermodynamics of the
situation, this type of approach is very sensitive.
Related Topics