Chapter: Psychology: Perception
The Neuroscience of Vision
THE NEUROSCIENCE OF VISION
Where are we so far? We started
by acknowledging the complexities of form perception— including the perceiver’s
need to interpret and organize visual information. We then con-sidered how this
interpretation might be achieved—through a network of detectors, shaped by an
interplay between bottom-up and top-down processes. But these points simply
invite the next question: How does the nervous system implement these
processes? More broadly, what events in the eye, the optic nerve, and the brain
make perception possible?
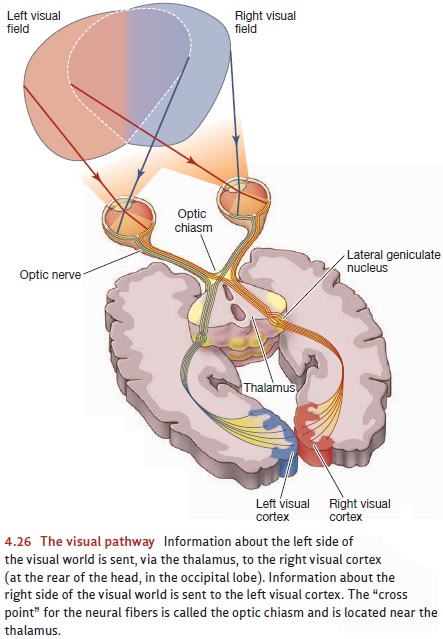
The Visual Pathway
As we saw, the rods and cones
pass their signals to the bipolar cells, which relay them to the ganglion cells
(Figure 4.26). The axons of the ganglion cells form the optic nerve, which
leaves the eyeball and begins the journey toward the brain. But even at this
early stage, the neurons are specialized in important ways, and different cells
are responsible for detecting different aspects of the visual world.
The ganglion cells, for example,
can be broadly classified into two categories: the smaller ones are called parvo cells, and the larger are called magno cells (parvo and magno are the
Latin for “small” and “large”). Parvo cells, which blanket the entireretina,
far outnumber magno cells. Magno cells, in contrast, are found largely in the
retina’s periphery. Parvo cells appear to be sensitive to color differences (to
be more pre-cise, to differences either in hue or in brightness), and they
probably play a crucial role in our perception of pattern and form. Magno
cells, on the other hand, are insensitive to hue differences but respond
strongly to changes in brightness; they play a central role in the detection of
motion and the perception of depth.
This pattern of neural
specialization continues and sharpens as we look more deeply into the nervous
system. The relevant evidence comes largely from the single-cell recording
technique that lets investigators determine which specific stimuli elicit a
response from a cell and which do not . This technique has allowed
investigators to explore the visual system cell by cell and has given us a rich
understand-ing of the neural basis for vision.
PARALLEL PROCESSING IN THE VISUAL CORTEX
We noted that cells in the visual
cortex each seem to have a “preferred stimulus”—and each cell fires most
rapidly whenever this special stimulus is in view. For some cells, the
preferred stimulus is relatively simple—a curve, or a line tilted at a particular
orientation. For other cells, the preferred stimulus is more complex—a corner,
an angle, or a notch. Still other cells are sensitive to the color (hue) of the
input. Others fire rapidly in response to motion—some
cells are particularly sensitive to left-to-right motion, others to the
reverse.
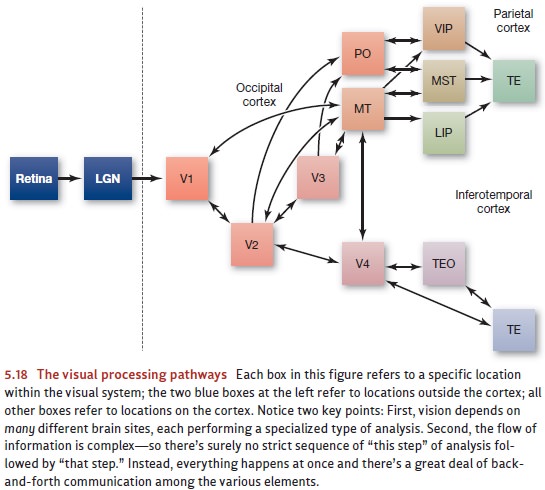
This abundance of cell types
suggests that the visual system relies on a “divide-and-conquer” strategy.
Different cells—and even different areas of the brain—each specialize in a
particular kind of analysis. Moreover, these different analyses go on in
parallel: The cells analyzing the forms do their work at the same time that
other cells are analyzing the motion and still others the colors. Using
single-cell recording, investigators have been able to map where these various
cells are located in the visual cortex as well as how they communicate with
each other; Figure 5.18 shows one of these maps.
Why this reliance on parallel
processing? For one thing, parallel processing allows greater speed, since (for
example) brain areas trying to recognize the shape of the stimulus aren’t kept
waiting while other brain areas complete the motion analysis or the color
analysis. Instead, all types of analysis can take place simultaneously. Another
advantage of parallel processing lies in its ability to allow each system to
draw informa-tion from the others. Thus, your understanding of an object’s
shape can be sharpened by a consideration of how the object is moving; your
understanding of its movement can be sharpened by noting what the shape is
(especially the shape in three dimen-sions). This sort of mutual influence is
easy to arrange if the various types of analysis are all going on at the same
time (Van Essen & DeYoe, 1995).

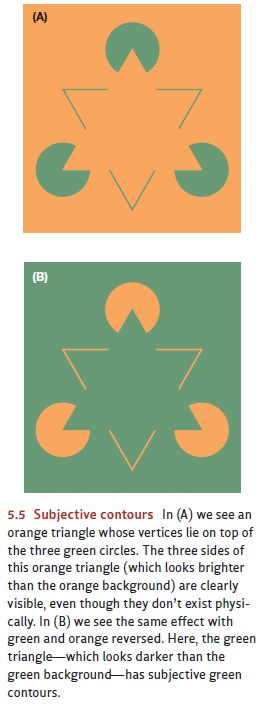
The parallel processing we see in
the visual cortex also clarifies a point we’ve already discussed. Earlier, we
argued that the inventory of a figure’s features depends on how the perceiver
organizes the figure. So, in Figure 5.4, the features of the letters were absent
with one interpretation of the shapes, but they’re easily visible with a
different interpretation. In Figure 5.5, some of the features (the triangle’s
sides) aren’t present in the figure but seem to be created by the way we
interpret the arrangement of features. Observations like these make it sound
like the interpretation has priority because it determines what features are
present.
But it would seem that the
reverse claim must also be correct because the way we inter-pret a form depends
on the features we can see. After all, no matter how you try to inter-pret
Figure 5.18, it’s not going to look like a race car, or a map of Great Britain,
or a drawing of a porcupine. The form doesn’t include the features needed for
those interpretations; and as a result, the form will not allow these
interpretations. This seems to suggest that it’s the features, not the
interpretation, that have priority: The features guide the interpre-tation, and
so they must be in place before the interpretation can be found.
How can both of these claims be
true—with the features depending on the interpreta-tion and the interpretation
depending on the features? The answer involves parallel pro-cessing. Certain
brain areas are sensitive to the input’s features, and the cells in these areas
do their work at the same time that other brain areas are analyzing the
larger-scale config-uration. These two types of analysis, operating in
parallel, can then interact with each other, ensuring that our perception makes
sense at both the large-scale and fine-grained levels.
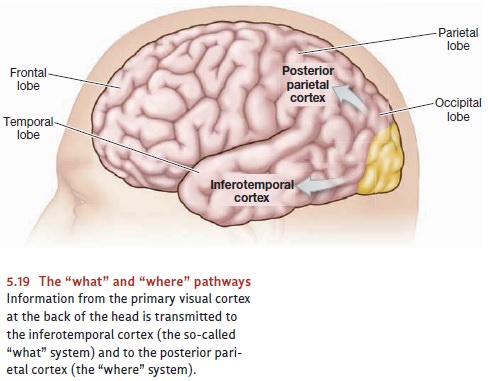
THE “ WHAT ” AND “ WHERE ” SYSTEMS
Evidence for specialized neural
processes, all operating in parallel, continues as we move beyond the visual
cortex. As Figure 5.19 indicates, information from the visual cortex is
transmitted to two other important brain areas in the inferotemporal cortex
(the lower part of the temporal cortex) and the parietal cortex. The pathway
carrying information to the temporal cortex is often called the “what” system; it plays a major role in
the identification of visual objects, telling us whether the object is a cat,
an apple, or whatever. The second pathway, which carries information to the
parietal cortex, is often called the “where”
system; it tells us where an object is located— above or below, to our
right or left (Ungerleider & Haxby, 1994; Ungerleider & Mishkin, 1982).
There’s been some controversy,
however, over how exactly we should think about these two systems. Some
theorists, for example, propose that the path to the parietal cortex isn’t
concerned with the conscious perception of position. Instead, it’s primarily
involved in the unnoticed, automatic registration of spatial location that
allows us to control our movements as we reach for or walk toward objects in
our visual world. Likewise, this view proposes that the pathway to the temporal
cortex isn’t really a “what” system; instead, it’s associated with our
conscious sense of the world around us, including our conscious recognition of
objects and our assessment of what these objects look like (e.g., Goodale &
Milner, 2004; also D. Carey, 2001; Sereno & Maunsell, 1998).
No matter how this debate is settled, there can be no question that these two path-ways serve very different functions. Patients who have suffered lesions in the occipi-tal-temporal pathway—most people agree this is the “what” pathway—show visual agnosia . They may be unable to recognize common objects, such as a cup or a pencil. They’re often unable to recognize the faces of relatives and friends— but if the relatives speak, the patients can recognize them by their voices. At the same time, these patients show little disorder in visual orientation and reaching. On the other hand, patients who have suffered lesions in the occipital-parietal pathway— usually understood as the “where” pathway—show the reverse pattern. They have difficulty in reaching for objects but no problem with identifying them (A. Damasio, Tranel, & H. Damasio, 1989; Farah, 1990; Goodale, 1995; Newcombe, Ratcliff, & Damasio, 1987).
The Binding Problem
It’s clear, then, that natural
selection has favored a division-of-labor strategy for vision: The processes of
perception are made possible by an intricate network of subsystems, each
specialized for a particular task and all working together to create the final
product—an organized and coherent perception of the world.
We’ve seen the benefits of this
design, but the division-of-labor setup also creates a problem for the visual
system. If the different aspects of vision—the perception of shape, color,
movement, and distance—are carried out by different processing mod-ules, then
how do we manage to recombine these pieces of information into one whole? For
example, when we see a ballet dancer in a graceful leap, the leap itself is
registered by the magno cells; the recognition of the ballet dancer depends on
parvo cells. How are these pieces put back together? Likewise, when we reach
for a coffee cup but stop midway because we see that the cup is empty, the
reach itself is guided by the occipital-parietal system (the “where” system);
the fact that the cup is empty is perceived by the occipital-temporal system
(the “what” system). How are these two streams of process-ing coordinated?
We can examine the same issue in
light of our subjective impression of the world around us. Our impression, of
course, is that we perceive a cohesive and organized world. After all, we don’t
perceive big and blue and distant; we instead perceive sky. We don’t perceive
brown and large shape on top of four shapes and moving; instead, we perceive
our pet dog running along. Somehow, therefore, we do manage to re-integrate the
separate pieces of visual information. How do we achieve this reunification?
Neuroscientists call this the binding
problem—how the nervous system manages to bind together elements that were
initially detected by separate systems.
We’re just beginning to
understand how the nervous system solves the binding problem. But evidence is
accumulating that the brain uses a pattern of neuralsynchrony—different
groups of neurons firing in synchrony with each other—toidentify which sensory
elements belong with which. Specifically, imagine two groups of neurons in the
visual cortex. One group of neurons fires maximally whenever a vertical line is
in view. Another group of neurons fires maximally whenever a stimulus is in
view moving from left to right. Also imagine that, right now, a vertical line
is presented, and it is moving to the right. As a result, both groups of
neurons are firing rapidly. But how does the brain encode the fact that these
attributes are bound together, different aspects of a single object? How does
the brain differentiate between this stimulus and one in which the features
being detected actually belong to different objects—perhaps a static vertical
and a moving diagonal?
The answer lies in the timing of
the firing by these two groups of neurons. We emphasized the firing rates of various neurons—whether a
neuron was firing at, say, 100 spikes per second or 10. But we also need to
consider exactly when a neuron is
firing, and whether, in particular, it is firing at the same moment as other
neurons. When the neurons are synchronized, this seems to be the nervous
system’s indication that the messages from the synchronized neurons are in fact
bound together. To return to our example, if the neurons detecting a vertical
line are firing in synchrony with the neurons signaling movement—like a group
of drummers all keep-ing the same beat—then these attributes, vertical and
moving, are registered as belong-ing to the same object. If the neurons are not
firing in synchrony, the features are registered as belonging to separate
objects (Buzsáki & Draguhn, 2004; Csibra, Davis, Spratling, & Johnson,
2000; M. Elliott & Müller, 2000; Fries, Reynolds, Rorie, & Desimone,
2001; Gregoriou, Gotts, Zhou, & Desimone, 2009).
Related Topics