Chapter: Basic & Clinical Pharmacology : Nonsteroidal Anti-Inflammatory Drugs, Disease-Modifying Antirheumatic Drugs, Nonopioid Analgesics,& Drugs Used in Gout
TNF-α-Blocking Agents
TNF-α-BLOCKING AGENTS
Cytokines play a
central role in the immune response and
in rheumatoid arthritis. Although a wide range of cytokines are expressed in
the joints of rheumatoid arthritis patients, TNF-α appears to be particularly important in the
inflammatory process.
TNF-α affects cellular
function via activation of specific membrane-bound TNF receptors (TNFR1, TNFR2). Five biologic
DMARDs interfering with TNF-α have been approved for the treatment of
rheumatoid arthritis and other rheumatic diseases (Figure 36–4).
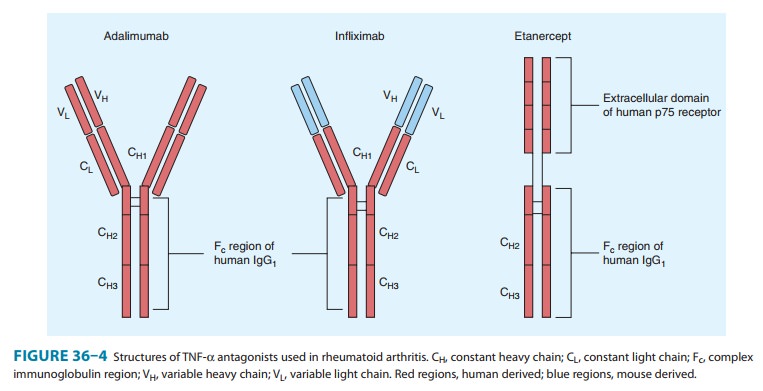
Adalimumab
A. Mechanism of Action
Adalimumab is a fully human IgG1 anti-TNF monoclonal anti-body. This compound complexes with soluble TNF-α and prevents its interaction with p55 and p75 cell surface receptors. This results in down-regulation of macrophage and T-cell function.
B. Pharmacokinetics
Adalimumab is given
subcutaneously and has a half-life of 10–20 days. Its clearance is decreased by
more than 40% in the presence of methotrexate, and the formation of human
anti-monoclonal antibody is decreased when methotrexate is given at the same
time. The usual dose in rheumatoid arthritis is 40 mg every other week;
increased responses may be evident with the higher weekly dosing regimen. In
psoriasis, 80 mg is given at week 0, 40 mg at week 1, and then 40 mg every
other week thereafter.
C. Indications
The
compound is approved for rheumatoid arthritis, ankylosing spondylitis,
psoriatic arthritis, juvenile idiopathic arthritis, plaque psoriasis, and
Crohn’s disease. It decreases the rate of formation of new erosions. It is
effective both as monotherapy and in combina-tion with methotrexate and other
DMARDs.
D. Adverse Effects
In common with the
other TNF-α-blocking
agents, the risk of bacterial infections and macrophage-dependent infection
(including tuberculosis and other opportunistic infections) is increased,
although it remains very low. Patients should be screened for latent or active
tuberculosis before starting adalimumab or other TNF-α-blocking agents. There is no evidence of an
increased incidence of solid malignancies. It is not clear if the incidence of
lymphomas is increased by adalimumab. A low incidence of newly formed dsDNA
antibodies and antinuclear antibodies (ANAs) has been documented when using
adalimumab, but clinical lupus is extremely rare. Rare cases of leukopenia and
vasculitis associated with adalimumab have been documented.
Certolizumab
A. Mechanism of Action
Certolizumab
is a recombinant, humanized antibody Fab frag-ment conjugated to a polyethylene
glycol (PEG) with specificity for human TNF-α. Certolizumab neutralizes
membrane-bound and soluble TNF-α in a dose-dependent manner.
Additionally, certolizumab does not contain an Fc
region, found on a complete antibody, and does not fix complement or cause
antibody-dependent cell-mediated cytotoxicity in vitro.
B. Pharmacokinetics
Certolizumab
is given subcutaneously and has a half-life of 14 days. The clearance is
decreased with decreasing body weight. Methotrexate does not alter the
pharmacokinetics of certolizumab. However, methotrexate does decrease the
appearance of anti-certolizumab antibodies. The usual dose for rheumatoid
arthritis is 400 mg ini-tially and at weeks 2 and 4, followed by 200 mg every
other week.
C. Indications
Certolizumab
is indicated for the treatment of adults with moder-ately to severely active
rheumatoid arthritis. It can be used as mono-therapy or in combination with
nonbiologic DMARDs. Additionally, certolizumab is approved to reduce signs and
symptoms and main-tain clinical response in adult patients with Crohn’s disease.
D. Adverse Effects
Consistent with other
TNF-α
blockers, the risk of serious infec-tions, including tuberculosis, fungal, and
other opportunistic pathogens, is increased and patients should be monitored
closely. Prior to initiation of treatment, testing for latent tuberculosis
should be performed. The association of lymphoma and other tumors with TNF-α blockers as a class,
of which certolizumab is a member, is not fully understood.
Etanercept
A. Mechanism of Action
Etanercept is a
recombinant fusion protein consisting of two sol-uble TNF p75 receptor moieties
linked to the Fc portion of human IgG1 (Figure 36–4); it
binds TNF-α
molecules and also inhibits lymphotoxin-α.
B. Pharmacokinetics
Etanercept
is given subcutaneously in a dosage of 25 mg twice weekly or 50 mg weekly. In
psoriasis, 50 mg is given twice weekly for 12 weeks followed by 50 mg weekly.
The drug is slowly absorbed, with peak concentration 72 hours after drug
administration. Etanercept has a mean serum elimination half-life of 4.5 days.
Fifty milligrams given once weekly gives the same area under the curve and
minimum serum concentrations as 25 mg twice weekly.
C. Indications
Etanercept
is approved for the treatment of rheumatoid arthritis, juvenile chronic
arthritis, psoriasis, psoriatic arthritis, and ankylo-sing spondylitis. It can
be used as monotherapy, although over 70% of patients taking etanercept are
also using methotrexate. Etanercept decreases the rate of formation of new
erosions relative to methotrexate alone. It is also being used in other
rheumatic syndromes such as scleroderma, Wegener’s granulomatosis, giant cell
arteritis, and sarcoidosis.
D. Adverse Effects
The
incidence of bacterial infections is slightly increased, especially soft tissue
infections and septic arthritis. Activation of latent tuber-culosis is lower
with etanercept than with other TNF-blocking agents. Nevertheless, patients
should be screened for latent or active tuberculosis before starting this
medication. Similarly, opportunistic infections can rarely occur when using
etanercept. The incidence of solid malignancies is not increased, but as with
other TNF-blocking agents, one must be aware of possible lymphomas (although
their incidence may not be increased compared with other DMARDs or active
rheumatoid arthritis itself ). While positive ANAs and dsDNAs may be found in
patients receiving this drug, these find-ings do not contraindicate continued
use if clinical lupus symptoms do not occur. Injection site reactions occur in
20–40% of patients, although they rarely result in discontinuation of therapy.
Anti-etanercept antibodies are present in up to 16% of treated patients, but
they do not interfere with efficacy or predict toxicity.
Golimumab
A. Mechanism of Action
Golimumab
is a human monoclonal antibody with a high affinity for soluble and
membrane-bound TNF-α.
Golimumab effectively neutralizes the inflammatory effects produced by TNF-α seen in
diseases such as rheumatoid arthritis.
B. Pharmacokinetics
Golimumab is
administered subcutaneously and has a half-life of approximately 14 days.
Concomitant use with methotrexate showed increased serum levels of golimumab as
well as a decrease in anti-golimumab antibodies. The recommended dose is 50 mg
given every 4 weeks.
C. Indications
Golimumab, given with
methotrexate, is indicated for the treat-ment of moderately to severely active
rheumatoid arthritis in adult patients. It is also indicated for the treatment
of psoriatic arthritis and ankylosing spondylitis.
D. Adverse Events
TNF-α blockers, including
golimumab, increase the risk of seri-ous infections, including tuberculosis,
fungal, and other opportu-nistic pathogens. Prior to initiation of treatment,
testing for latent tuberculosis should be performed. As with other TNF-α-blocking agents,
there is a potential association with lymphoma; there is no association with
other solid tumors (except possibly non-melan-otic skin cancers).
Infliximab
A. Mechanism of Action
Infliximab
(Figure 36–4) is a chimeric (25% mouse, 75% human) IgG1
monoclonal antibody that binds with high affinity to soluble and possibly
membrane-bound TNF-α.
Its mechanism of action probably is the same as that of adalimumab.
B. Pharmacokinetics
Infliximab is given as
an intravenous infusion with “induction” at 0, 2, and 6 weeks and maintenance
every 8 weeks thereafter. Dosing is 3–10 mg/kg, although the usual dose is 3–5
mg/kg every 8 weeks. There is a relationship between serum concentra-tion and
effect, although individual clearances vary markedly. The terminal half-life is
9–12 days without accumulation after repeated dosing at the recommended
interval of 8 weeks. After intermittent therapy, infliximab elicits human
antichimeric antibodies in up to 62% of patients. Concurrent therapy with
methotrexate markedly decreases the prevalence of human antichimeric
antibodies.
C. Indications
Infliximab is approved
for use in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis,
and Crohn’s disease. It is being used in other diseases, including psoriasis,
ulcerative colitis, juvenile chronic arthritis, Wegener’s granulomatosis, giant
cell arteritis, and sarcoidosis. In rheumatoid arthritis, a regimen of
infliximab plus methotrexate decreases the rate of formation of new erosions
more than methotrexate alone over 12–24 months. Although it is recom-mended
that methotrexate be used in conjunction with infliximab, a number of other
DMARDs, including antimalarials, azathioprine, leflunomide, and cyclosporine,
can be used as background therapy for this drug. Infliximab is also used as
monotherapy, although this is neither approved by regulatory agencies nor advisable.
D. Adverse Effects
Like other TNF-α-blocking agents,
infliximab is associated with an increased incidence of bacterial infections,
including upper respiratory tract infections. As a potent macrophage inhibitor,
infliximab can be associated with activation of latent tuberculosis, and patients
should be screened for latent or active tuberculosis before starting therapy.
Other infections have been documented, though rarely. There is no evidence of
an increased incidence of solid malignancies and it is not clear whether the
incidence of lymphoma is increased with infliximab. Because rare demyelinat-ing
syndromes have been reported, patients with multiple sclerosis or neuro-uveitis
should not use infliximab. Rare cases of leukope-nia, hepatitis, activation of
hepatitis B, and vasculitis have been documented. The incidence of positive ANA
and dsDNA anti-bodies is increased, although clinical lupus erythematosus
remains an extremely rare occurrence and the presence of ANA and dsDNA does not
contraindicate the use of infliximab. Infusion site reactions correlate with
anti-infliximab antibodies. These reactions occur in approximately 3–11% of
patients, and the com-bined use of antihistamines and H2-blocking agents
apparently prevents some of these reactions.
Related Topics